While Awaiting Lyme Disease Vax, Treatment Reduction Offers Benefits
Next-generation Lyme Disease treatments and vaccines continue to be in high demand in Europe and the U.S. As the geographic footprint of this vector-borne illness widens, it is estimated that 476,000 people in the U.S. and 130,000 people in Europe are impacted each year.
Recent news announced by New York-based Pfizer Inc. and France-based Valneva SE confirmed their investigational Lyme disease vaccine candidate VLA15 is progressing with a late-stage clinical trial.
Pending successful completion of the Phase 3 study, Pfizer could potentially submit a Biologics License Application to the U.S. Food and Drug Administration (FDA) and Marketing Authorisation Application to the European Medicines Agency in 2025.
Until then, treating Lyme disease infections remains a top priority.
Researchers from Solvaneia recently reported in The Lancet Infectious Diseases how to reduce potentially harmful antibiotic overuse by identifying shorter effective treatments.
This clinical study aimed to assess whether oral doxycycline for seven days was non-inferior to 14 days of treatment in adults with solitary erythema migrans.
In this randomized open-label non-inferiority trial, they enrolled about 300 patients with solitary erythema who were given doxycycline 100 MG Oral Tablet at the University Medical Centre in Ljubljana, Slovenia.
Five of 147 patients from the 7-day group versus 3 of 147 patients from the 14-day group had treatment failure manifesting as persistence of erythema (difference 1.4%; upper limit of one-sided 95% CI 5.2%; p=0·64).
This study's data supports the reduced seven days of treatment with oral doxycycline for patients with solitary erythema migrans.
The benefit of this finding is reducing antibiotic exposure to the current guideline-driven therapy.
The researchers wrote, "The 7-day regimen appears to fulfill the fundamental treatment goals of preventing progression to more advanced Lyme disease while limiting antibiotic exposure."
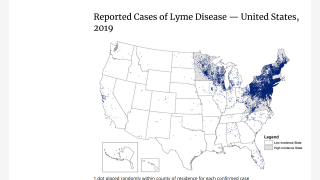
The U.S. CDC says Lyme disease is generally caused by the bacterium Borrelia burgdorferi.
Left untreated, the disease can disseminate and cause more serious complications affecting the joints, the heart, or the nervous system.
Lyme disease is transmitted to humans through the bite of infected black-legged ticks.
Until a Lyme disease vaccine is approved, the steps to prevent Lyme disease include using insect repellent, removing ticks promptly, applying pesticides, and reducing tick habitat.
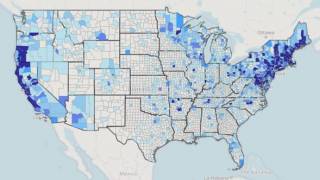
A recent study by FAIR Health found a significant increase in Lyme disease diagnoses over the past 15 years in the U.S.
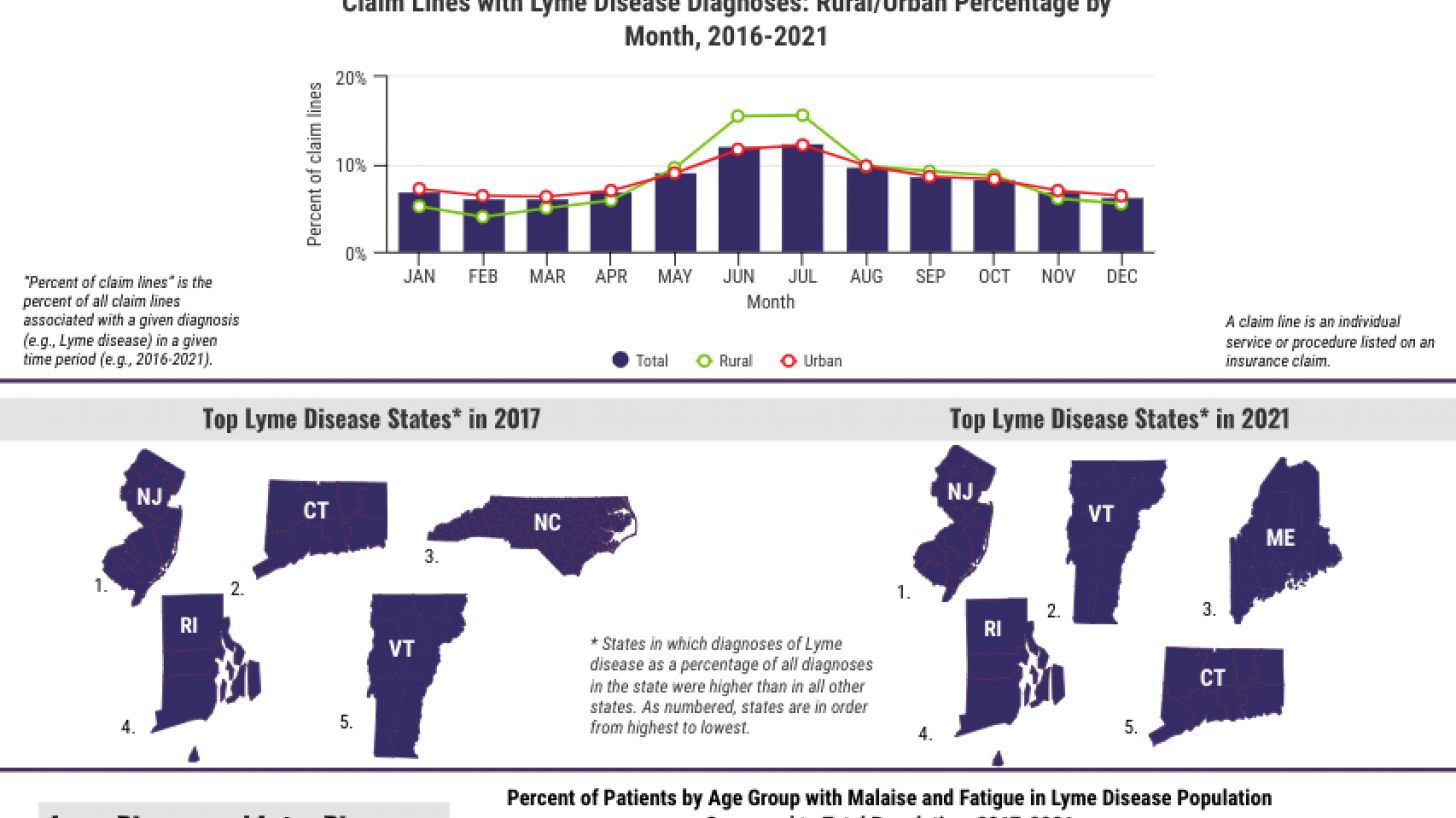
Results of the study published on August 5, 2022, indicate that from 2007 to 2021, private insurance claim lines with Lyme disease diagnoses increased by 357% in rural areas and 65% in urban areas.
In 2021, the top U.S. states were New Jersey, Vermont, Maine, Rhode Island, and Connecticut.
As of October 10, 2022, the FDA had not approved any Lyme disease vaccine.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually translated and curated for mobile readership.
Our Trust Standards: Medical Advisory Committee