Who is Infected With Tick-borne Lyme Disease
Lyme disease has continued to spread worldwide, but there’s little scientific agreement on how common is this tick-borne disease.
To resolve this knowledge gap, the open-access journal BMJ Global Health revealed a pooled data analysis on June 13, 2022, that found more than 14% of the world’s population probably has or has had the presence of antibodies to Borrelia burgdorferi sensu lato (Bb) infection in the blood.
The three regions with the highest reported seroprevalence were Central Europe (21%), Eastern Asia (16%), and Western Europe (13.5%).
At the other end of the scale, the regions with the lowest reported seroprevalence were the Caribbean (2%), Southern Asia (3%), and Oceania (nearly 5.5%).
A smaller pooled analysis of the results of 58 different studies showed that older age (50+), men, residence in a rural area, and being bitten by a tick were all associated with a heightened risk of Bb antibodies.
“Our results indicate that the prevalence of Bb in 2010–2021 was higher than that in 2001–2010,” wrote the study’s authors.
However, the study authors conclude: “The reported estimated global Bb seropositivity is relatively high….[Lyme disease] is a widely distributed infectious disease but has not received much attention worldwide.”
“A more accurate picture of its global distribution and who is most at risk of infection could inform the development of public health response policies and [Lyme disease] control programs,” they suggest.
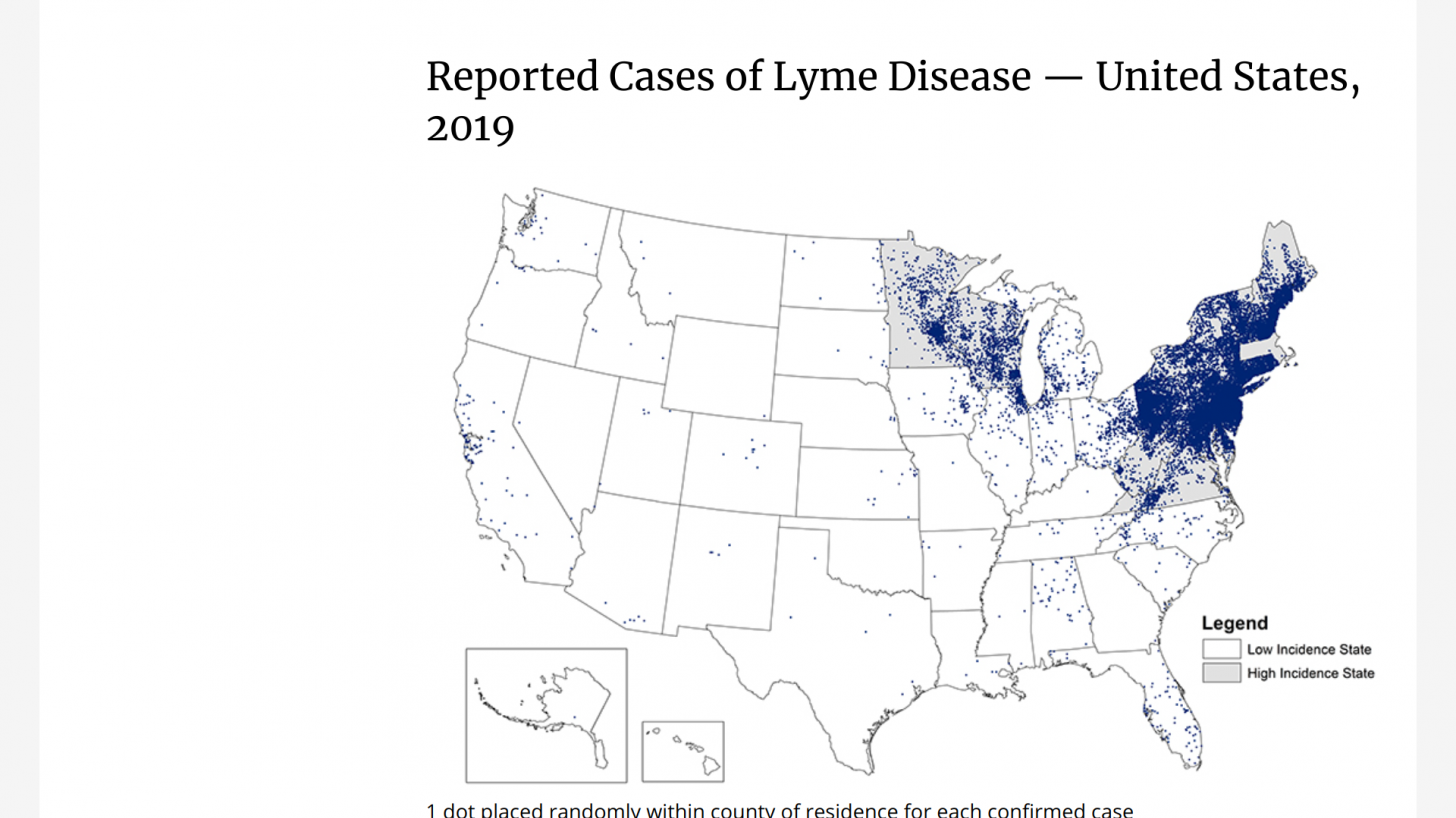
The U.S. CDC says, ‘There is no way of knowing exactly how many people get Lyme disease.’
A recently released estimate based on insurance records suggests that each year approximately 476,000 Americans are diagnosed and treated for Lyme disease. Regardless, this number indicates a significant burden on the healthcare system and the need for more effective prevention measures.
While the U.S. FDA has not approved a Lyme disease preventive vaccine, one candidate is conducting a late-stage, phase 3 clinical study.
France-based Valneva SE vaccine candidate VLA15 is a multivalent recombinant protein vaccine targeting the outer surface protein A of Borrelia, aiming for protection against most human pathogenic Borrelia species.
Valneva and New York-based Pfizer, Inc. announced a collaboration for VLA15's development and commercialization in April 2020.
On February 4, 2022, Valneva and Pfizer announced a three-dose primary series vaccination schedule in a planned Phase 3 clinical trial with adults and pediatric subjects and is expected to be initiated in the third quarter of 2022, subject to regulatory approval.
Kathrin U. Jansen, Ph.D., Senior Vice President and Head of Vaccine Research & Development at Pfizer, commented in a press release issued on April 26, 2022, “The medical need for vaccination against Lyme disease is steadily increasing as the geographic footprint of the disease widens.”
“These positive pediatric data mark an important step forward in the ongoing development of VLA15, and we are excited to continue working with Valneva to potentially help protect both adults and children from Lyme disease.”
Note: No researcher industry conflicts of interest were disclosed. Dong Y, Zhou G, Cao W, et al. Global seroprevalence and sociodemographic characteristics of Borrelia burgdorferi sensu lato in human populations: a systematic review and meta-analysis BMJ Global Health 2022;7:e007744.
Professor Fukai Bao; baofukai@kmmu.edu.cn.
PrecisionVaccinations publishes fact-checked, research-based vaccine news that is curated for mobile readership.
Our Trust Standards: Medical Advisory Committee