Can Vaccinations Resolve Tuberculosis Disruptions

With the theme "Yes! We Can End TB: Commit, Invest, Deliver," World Tuberculosis Day 2025 highlights a rallying cry for urgency, accountability, and hope.
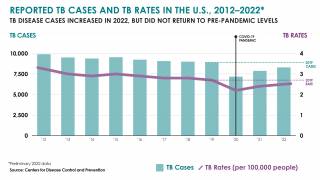
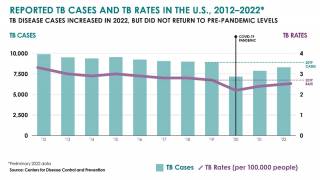
Scheduled for March 24, 2025, World Tuberculosis Day 2025 activities amplify the urgency of ending tuberculosis outbreaks, the world's deadliest infectious disease, responsible for over 1 million people annually, devastatingly impacting families and communities.
The WHO says global efforts to combat tuberculosis (TB) have saved an estimated 79 million lives since 2000. However, reductions in global health funding are threatening to reverse these gains.
Dr Tedros Adhanom Ghebreyesus, WHO Director-General, stated in a press release, "But we cannot give up on the concrete commitments that world leaders made at the UN General Assembly just 18 months ago to accelerate work to end TB. WHO is committed to working with all donors, partners, and affected countries to mitigate the impact of funding cuts and find innovative solutions."
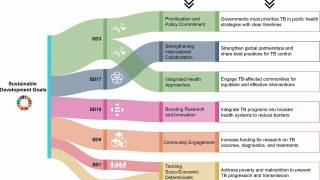
The WHO Global Tuberculosis Report 2024 says TB is a vaccine-preventable disease.
The WHO has stated that increasing access to TB vaccines could save up to 1.9 billion antibiotic doses annually, helping reduce antimicrobial resistance.
While versions of the Bacille Calmette-Guérin (BCG) vaccine have been used for about 100 years and over 4 billion completed vaccinations, it has demonstrated only 50% effectiveness.
Unfortunately, the WHO has not issued a universal BCG vaccination policy.
To fill this void, about 15 different TB vaccines are currently used in various countries, with localized vaccination guidelines.
For example, access to the BCG vaccines has been minimal in the United States.
An article published by IJID Regions (Volume 14) on March 2, 2025, underscores that achieving TB elimination requires vaccines to prevent TB infection reactivation and transmission of drug-resistant strains.
For certain cancer patients, U.S. policy recently changed.
As of March 13, 2025, the Serum Institute of India and ImmunityBio's recombinant Bacillus Calmette-Guérin (rBCG) became available in the U.S. for bladder cancer therapies.
The rBCG vaccine has two gene modifications to improve its immunogenicity and safety. Compared to earlier BCG vaccine formulations, it has demonstrated potent immunogenicity with CD8+ and CD4+ T-cell stimulation.
As of March 22, 2025, this rBCG vaccine is available for bladder cancer therapies.
Previously, Merck's TICE® BCG was the only TB vaccine available in the U.S., primarily offered at public health departments to prevent TB in children.
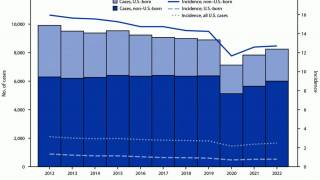
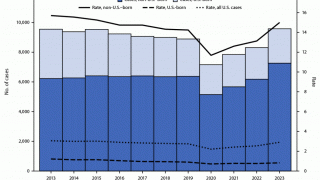
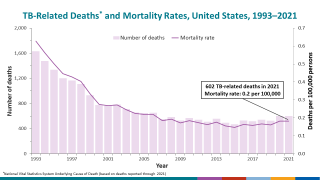
With the increasing number of TB cases in the U.S. and changes in healthcare policy, TB vaccinations may become a tool in reducing this infectious disease.
Our Trust Standards: Medical Advisory Committee