Potential On-Demand Lyme Disease Protection
Tarsus Pharmaceuticals, Inc. has just announced positive topline results from the Phase 2a Carpo trial evaluating TP-05 (lotilaner). This novel, oral therapeutic candidate has shown remarkable effectiveness in preventing Lyme disease.
TP-05 is not U.S. FDA-approved but is believed to be the only non-vaccine, drug-based, preventative therapeutic in development designed to kill ticks before they transmit Lyme disease to people.
This vector-borne disease often goes undetected because ticks are not always noticed.
In most cases, ticks must be attached for about 36-48 hours or more before Lyme disease can be transmitted, so quickly killing ticks can significantly increase the probability of disease prevention.
“Lyme disease remains a growing public health concern associated with long-term consequences for millions of patients and an estimated $1 billion dollar price tag for the U.S. healthcare system,” said Bobby Azamian, M.D., Ph.D., Chief Executive Officer and Chairman of Tarsus, in a press release on February 22, 2024.
“We are highly encouraged by these early proof-of-concept data and the opportunity to bring forward a novel, on-demand, oral treatment that addresses the root cause of disease – the ticks that transmit the bacteria that cause Lyme disease.”
TP-05 is an oral systemic formulation of lotilaner, a well-characterized anti-parasitic agent that selectively inhibits parasite-specific GABA-Cl channels. Both the high and low doses of TP-05 demonstrated a statistically significant benefit in killing ticks compared to placebo.
Specifically, after the Day 1 tick challenge, mean tick mortality was 97.0% (± 1.4 standard error, SE) and 92.0% (± 6.3 SE) for the high and low doses of TP-05, respectively, compared to only 5.0% (± 2.5 SE) for placebo (p < 0.0001).
Similarly, at the 30-day challenge, mean tick mortality 24 hours after placement was 89.0% (± 11.1 SE) and 91.0% (± 6.1 SE) for the high and low doses of TP-05, respectively, compared to only 9.0% (± 8.0 SE) for placebo (p<0.001).
No statistically significant differences in tick mortality were observed between the two TP-05 treatment arms, and TP-05 was generally well tolerated.
Linden Hu, M.D., the Paul and Elaine Chervinsky Professor in Immunology at Tufts University School of Medicine and Principal Investigator for the Carpo trial, commented: “The tick-kill rates seen at Day 1 and Day 30 suggest that TP-05 may have the potential to provide both rapid and durable protection against multiple tick-borne diseases, which would be a welcome alternative to vaccines in the prevention armamentarium.”
Lyme disease can potentially cause severe, often debilitating symptoms with permanent and irreversible damage.
The disease can result in inflammation, nerve, joint, and muscle pain and swelling, numbness, shortness of breath, and – in severe cases – neurological complications such as facial palsy, vision issues, and meningitis, including severe headaches and neck stiffness, as well as cardiac complications.
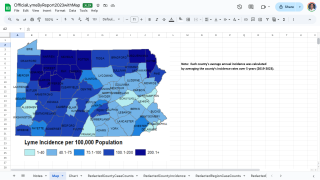
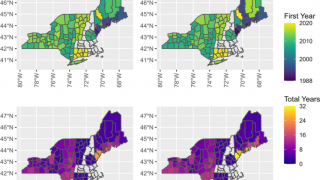
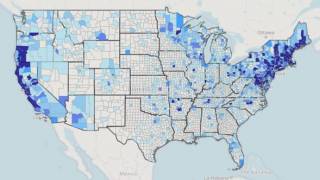
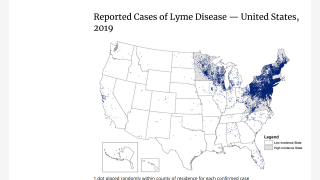
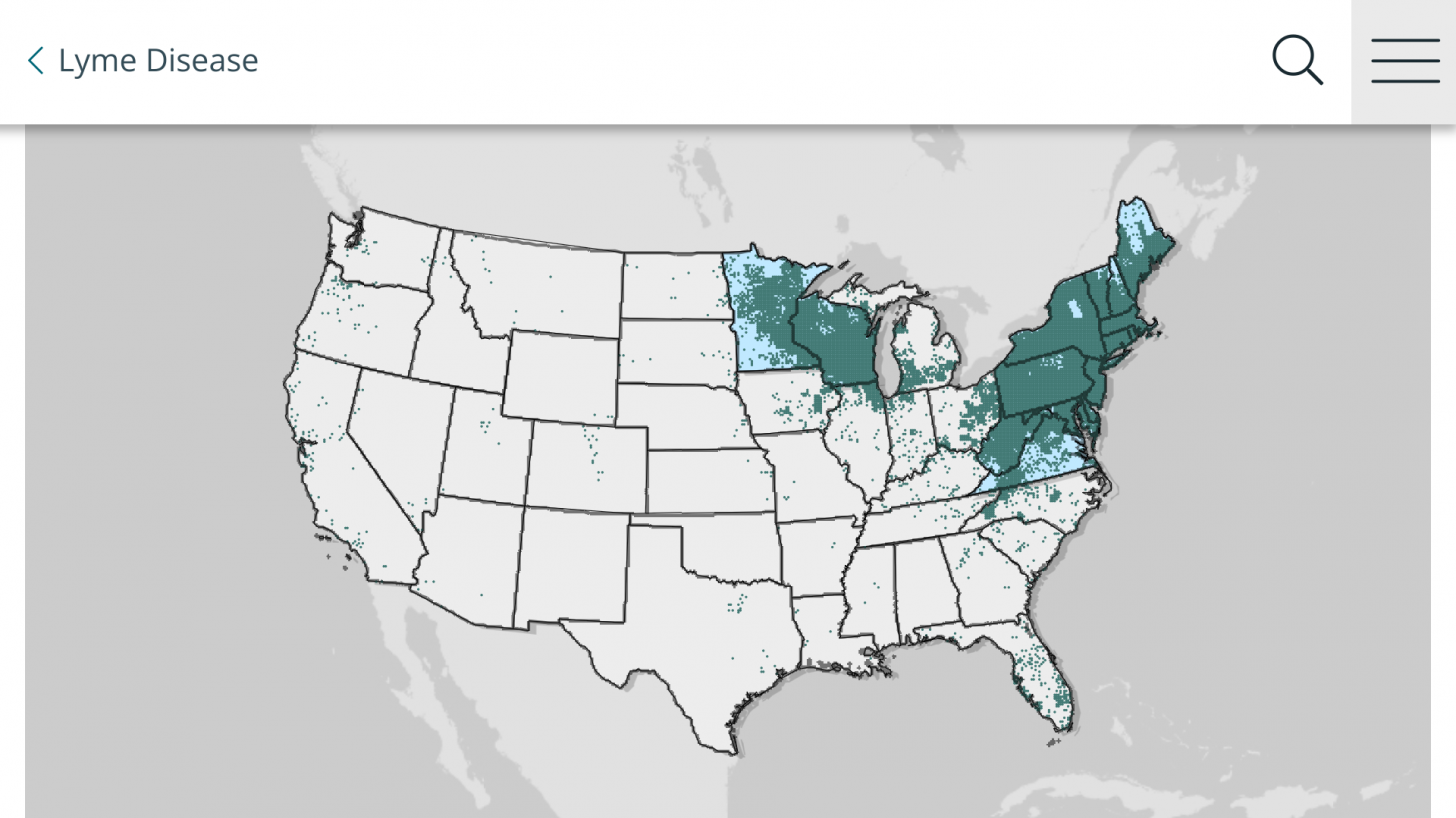
Data from the U.S. Centers for Disease Control and Prevention shows Lyme disease outbreaks increased by 69% during 2017–2019 in the United States. And the risk of Lyme disease spreading to new geographical areas.
As of 2022, high-incidence jurisdictions are Connecticut, Delaware, the District of Columbia, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, West Virginia, and Wisconsin.
As of February 2024, there are no FDA-approved pharmacological prophylactic options for Lyme disease.
However, an innovative Lyme disease vaccine candidate is conducting late-stage clinical research in 2024. VLA15 is a multivalent recombinant protein vaccine that protects humans by increasing antibodies that prevent Borrelia from migrating from ticks after a bite.
Pfizer recently indicated it could submit a Biologics License Application for VLA15 to the U.S. FDA as early as 2025.
Our Trust Standards: Medical Advisory Committee