Lyme Disease Vax Candidate Posts Positive Antibody Persistence Data
According to an announcement today, a Lyme disease vaccine candidate may soon obtain approval in the U.S. This would be significant since Lyme disease remains a serious health risk to most people.
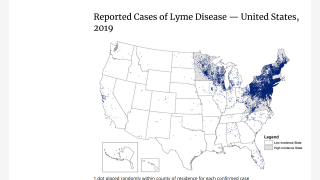
This vector-borne illness is considered common in the Northern Hemisphere.
Each year, approximately 30,000 cases of Lyme disease are reported to U.S. CDC.
Earlier today, Pfizer Inc. and Valneva SE reported antibody persistence data six months after completing a three-dose or a two-dose vaccination schedule with their Lyme disease vaccine candidate.
This is the first time antibody persistence data have been reported in pediatric populations for the VLA15 vaccine candidate.
VLA15 is the only multivalent protein subunit Lyme disease vaccine candidate currently in clinical development.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, stated in a press release on December 1, 2022, "We are pleased with these antibody persistence data that further validate the use of the three-dose vaccination schedule in our ongoing Phase 3 study and the acceptable safety and tolerability profiles of our vaccine candidate."
"Lyme disease continues to spread, representing a high unmet medical need that impacts the lives of many in the Northern Hemisphere, and each new report of positive data takes us a step closer to potentially bringing this vaccine to both adults and children who could benefit from it."
Earlier this year, Pfizer and Valneva initiated a Phase 3 clinical study, Vaccine Against Lyme for Outdoor Recreationists, to investigate the efficacy, safety, and immunogenicity of VLA15.
Approximately 6,000 participants five years of age and older will receive three doses of VLA15 180 µg or saline placebo as a primary vaccination series followed by one booster dose of VLA15 or saline placebo (1:1 ratio).
Enrollment is ongoing in Europe and the U.S. and is expected to be completed in the second quarter of 2023.
To achieve the required pediatric safety database, Pfizer and Valneva plan to initiate a complementary Phase 3 clinical study in early December 2022 to collect additional VLA15 safety data in participants 5 to 17.
"Rates of Lyme disease continue to increase globally, underscoring the importance of a vaccine that may help protect both adults and children," commented Annaliesa Anderson, Ph.D., SVP and Chief Scientific Officer of Vaccine Research & Development at Pfizer.
"These six-month antibody persistence data are encouraging, and we hope that the data generated from the Phase 3 studies will further support the positive evidence for VLA15 to date."
Pending successful completion of the Phase 3 studies, Pfizer could potentially submit a Biologics License Application to the U.S. FDA and Marketing Authorisation Application to the European Medicines Agency in 2025.
Lyme disease is a systemic infection caused by Borrelia burgdorferi bacteria transmitted to humans by infected Ixodes ticks.
While the true worldwide incidence of Lyme disease is unknown, but it is estimated to affect approximately 130,000 people in Europe.
Early symptoms of Lyme disease, such as a gradually expanding erythematous rash called Erythema migrans, or more nonspecific symptoms like fatigue, fever, headache, mild stiff neck, arthralgia, or myalgia, are often overlooked or misinterpreted, says the CDC.
Left untreated, the disease can disseminate and cause more serious complications affecting the joints, the heart, or the nervous system.
The medical need for vaccination against Lyme disease is steadily increasing as the geographic footprint of the disease widens.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee