Lyme Disease Vaccine Candidate Leverages mRNA Technology
New preclinical research from the Perelman School of Medicine at the University of Pennsylvania suggests that an innovative vaccine candidate can prevent developing Lyme disease in people.
While there are existing Lyme disease vaccines for dogs, no vaccine candidate is approved for people.
“Bacteria are more complex organisms than viruses, and therefore, it can be more challenging to develop effective vaccines against them,” said senior author Norbert Pardi, Ph.D., an assistant professor of Microbiology, in a press release on September 19, 2023.
“Here we were able to identify a target for a mRNA vaccine that shows promising results for preventing B. burgdorferi infection in animal models.”
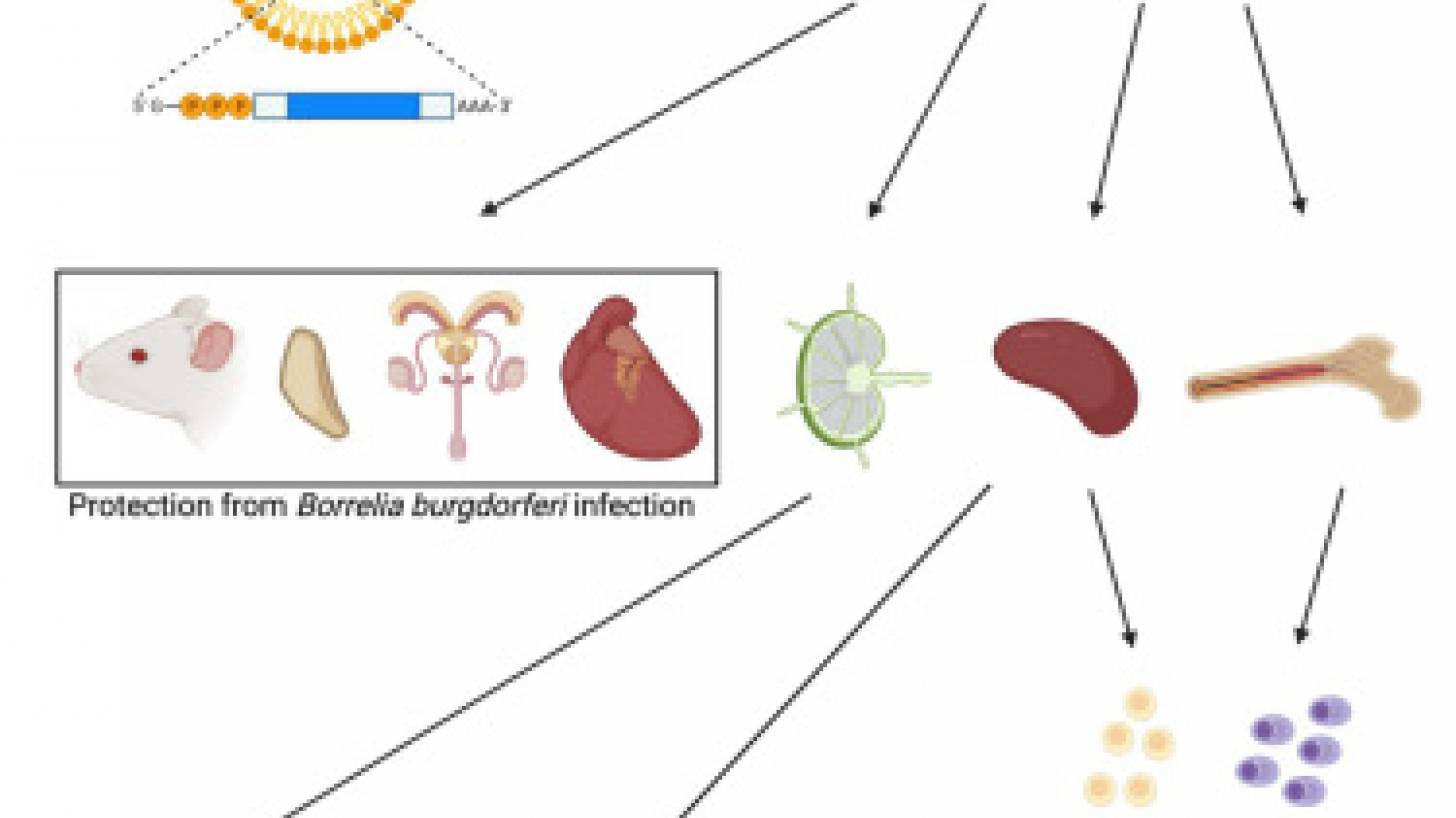
The vaccine, described in an Original Article published in the journal Cell Press on August 1, 2023, uses the same messenger ribonucleic acid (mRNA) technology pioneered at Penn.
They identified one of the proteins in B. burgdorferi that elicits a potent immune response called outer surface protein A (OspA).
OspA is a conserved protein in the multiple strains of B. burgdorferi, making it an ideal target for preventing an initial B. burgdorferi infection from progressing to Lyme disease.
These Penn researchers demonstrated that OspA mRNA-lipid nanoparticle (LNP) displayed superior immunogenicity and efficacy to an alum-adjuvanted OspA protein subunit vaccine when used at 3 μg and 1 μg dose, respectively.
First, mice immunized with a single dose of OspA mRNA-LNP elicited more robust polyfunctional CD8+ and CD4+ T cell responses than the OspA protein vaccine.
Though antibody response is the key to preventing B. burgdorferi infection in the host, anti-OspA CD8+ T cells could provide further immune support as spirochetes in some patients express OspA in late infection.
Second, one particular subset of CD4+ T cells, Tfh cell, and with antigen-specific GC B cells, were more greatly elevated in mice after a single vaccination with OspA mRNA-LNP than in mice immunized with rOspA + alum.
Increased magnitude of germinal center responses corresponded to increased levels of its terminal outputs, antigen-specific,c MBCs and LLPCs, which secreted IgG1, IgG2a, andIgG2b, after immunization with a single dose of OspA mRNA-LNP.
Third, a prime-boost regimen of OspA mRNA-LNP compared to one of rOspA + alum yielded higher, longer-lasting levels of OspA-specific antibodies, which are crucial to blocking the transmission of B. burgdorferi to the host from feeding ticks.
Most importantly, a single dose of OspA mRNA-Lly protected mice from B. burgdorferi infection successfully than its recombinant protein comparator.
Recombinant OspA has been previously shown to be fully protective in mice when administered at much higher doses and with multiple bo.
Still, this study utilizes a single low-dose regimen that offers partial protection.
Finally, although mice were challenged by needle injection in this study, an OspA mRNA-LNP vaccine could be highly effective against ticks challenge representing the natural infection route.
The immunogenicity and protective efficacy of OspA mRNA-LNP outperformed alum-adjuvanted recombinant OspA protein, which is analogous to the LYMErix vaccine released by GSK in 1998. The LYMErix vaccine was later discontinued.
With further preclinical and clinical development, these researchers wrote that OspA mRNA-LNP could prove to be a viable preventative approach to curtailing the pervasiveness of Lyme disease.
This research was funded by the National Institute of Allergy and Infectious Diseases (R01AI146101, R01AI153064, AI126033, AI165499, AI138949, R01AI142572, R21AI137433, R01AI153064, and R01AI168312), the Steven and Alexandra Cohen Foundation, the Howard Hughes Medical Institute Emerging Pathogens Program, and the Burroughs Wellcome Fund (BWF1012376).
Our Trust Standards: Medical Advisory Committee