France’s Lyme Disease Vaccine Candidate Continues Posting Positive Results

A France based specialty vaccine company announced positive initial results for its second Phase 2 study (VLA15-202) of Lyme disease vaccine candidate VLA15.
VLA15 is the only active Lyme disease vaccine in clinical development, and covers (6) serotypes that are prevalent in North America and Europe.
Valneva SE’s Juan Carlos Jaramillo, M.D., Chief Medical Officer, stated in a press release published on October 20, 2020, “We are extremely pleased with these results which showed an excellent immunological profile, further supported by additional positive data through the Serum Bactericidal Assay (SBA).”
“With these encouraging data, we are now well-positioned to continue vaccine development. Lyme disease continues to be a high unmet medical need and our objective remains to work closely with Pfizer to offer a preventative solution as soon as possible.”
Compared to study VLA15-201, study VLA15-202 investigated a vaccination schedule of Month 0-2-6 based on matching doses. And, VLA15 was generally safe across all doses and age groups tested.
The VLA15 vaccine candidate’s tolerability profile including fever rates was comparable to other lipidated recombinant vaccines or lipid-containing formulations.
Furthermore, as in VLA15-201, no related Serious Adverse Events (SAEs) were observed in any treatment group. Reactogenicity decreased following the first vaccination.
Compared to study VLA15-201, immunogenicity was further enhanced using a Month 0-2-6 schedule. The Seroconversion Rates (SCR), after completion of the primary vaccination series, showed similar responses and ranged from 93.8% [ST1] to 98.8% [ST2, ST4]. Antibody responses were comparable in the 2-dose groups tested.
The immunological response in older adults, one of the main target groups for a Lyme vaccine, is particularly encouraging, as already observed in VLA15-201.
Moreover, these new results did not indicate that prior exposure to Borrelia spirochetes (seropositivity) has an impact on immunogenicity or safety, also as observed in VLA15-201.
An SBA assessing the functional immune response against Lyme disease after vaccination with VLA15 was conducted for the first time and demonstrated the functionality of antibodies against all OspA serotypes.
Assays, such as SBAs, are commonly used to enable a potential prediction of vaccine efficacy via the measurement of vaccine-induced functional immune responses.
This investigational multivalent protein subunit vaccine VLA15 targets the outer surface protein A of Borrelia, an established mechanism of action for a Lyme disease vaccine. OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick.
Lyme disease Overview
Lyme disease is a systemic infection caused by Borrelia bacteria transmitted to humans by infected Ixodes ticks. It is considered the most common vector-borne illness in the Northern Hemisphere.
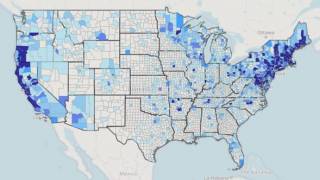
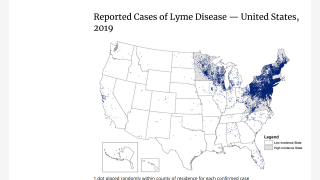
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 300,000 Americans are diagnosed with Lyme disease each year with the state of Pennsylvania reporting the most cases in 2018.
And, at least a further 200,000 cases were confirmed in Europe.
The number of counties with an incidence of ≥10 confirmed cases per 100,000 persons increased from 324 in 2008 to 415 in 2018, says the CDC.
Early symptoms of Lyme disease include a gradually expanding erythematous rash called Erythema migrans or more unspecific symptoms like fatigue, fever, headache, mild stiff neck, arthralgia, or myalgia, are often overlooked or misinterpreted.
Left untreated, the disease can disseminate and cause more serious complications affecting the joints, the heart, or the nervous system.
For more information, visit Valneva.
PrecisionVaccinations publishes research-based vaccine development news.
Our Trust Standards: Medical Advisory Committee