Lyme Disease Vaccine Gains Global Partner

Hikers concerned about their walk in the woods this summer may soon have access to a protective Lyme disease vaccine.
On April 30, 2020, a French specialty vaccine company announced a significant collaboration to commercialize the Lyme disease vaccine candidate VLA15, which is currently in Phase 2 clinical studies.
Previously, the Valneva SE VLA15 vaccine demonstrated strong immunogenicity and safety data in pre-clinical and Phase 1 studies.
Valneva’s collaboration with Pfizer Inc. indicates this protective vaccine could become available soon.
VLA15 is the only active Lyme disease vaccine program in clinical development today and covers 6 serotypes that are prevalent in North America and Europe.
The investigational multivalent protein subunit vaccine, VLA15, targets the outer surface protein A (OspA) of Borrelia, an established mechanism of action for a Lyme disease vaccine.
OspA is one of the most dominant surface proteins expressed by the bacteria when present in ticks, which often spread this bacteria.
Valneva has completed patient enrolment and follow-up for two Phase 2 studies of its Lyme disease vaccine candidate, in more than 800 people. Valneva expects to report the first Phase 2 results in mid-2020.
Under the terms of the agreement, Valneva is eligible to receive a total of $308 million in cash payments and will fund 30% of all development costs through completion of the development program.
In return, Pfizer will pay Valneva tiered royalties and will lead the late-stage development and have sole control over the future commercialization of the vaccine.
“Lyme disease is the most commonly reported tick-borne illness in the USA and is growing in its prevalence and geographic reach. We look forward to bringing a new solution to patients for this significant unmet need,” said Nanette Cocero, Global President, Pfizer Vaccines, in a related press release.
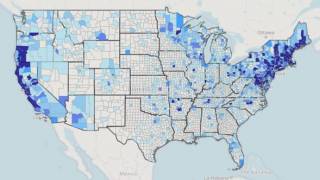
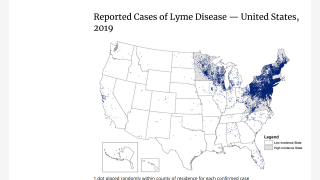
Lyme disease is a systemic infection caused by Borrelia bacteria transmitted to humans by infected Ixodes ticks. It is considered the most common vector-borne illness in the Northern Hemisphere.
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 300,000 Americans are diagnosed with Lyme disease each year with at least a further 200,000 cases in Europe.
Left untreated, the Lyme disease can disseminate and cause more serious complications affecting the joints (arthritis), the heart (carditis) or the nervous system.
The medical need for vaccination against Lyme disease is steadily increasing as the disease footprint widens, says the CDC.
The program was granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in July 2017.
Valneva is a specialty vaccine company focused on prevention against diseases with major unmet needs.
Lyme disease vaccine news published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee