First Chikungunya Vaccine Approved for Adolescents
For about 200 years, health organizations like the World Health Organization (WHO) have confirmed Chikungunya virus transmissions. In 2024, outbreaks reached an unfortunate level in the Region of the Americas.
Last year, 425,138 Chikungunya virus (CHIKV) cases and 236 associated fatalities were reported by the Pan American Health Organization. This year has started with over 55,000 cases as of early March.
The vast majority of the infections were in unvaccinated people.
To address this shortcoming, the U.S. Food and Drug Administration recently approved a novel Chikungunya vaccine produced by Bavarian Nordic A/S, which became commercially available on March 18, 2025.
This announcement means before international travelers plan their next trip to CHIKV-endemic areas such as Argentina and Brazil, the VIMKUNYA™ vaccine is available for adolescents and adults to help prevent the contraction of this mosquito-transmitted disease and the potential for long-term illness.
Peter Costa, Bavarian Nordic's Medical Director of North America, informed Vax-Before-Travel News on March 21, 2025, "that "With approved use for those 12 years of age and older, VIMKUNYA™ addresses an important unmet public health need by expanding the eligibility for vaccination against Chikungunya."
"As the first virus-like particle (VLP) single-dose, pre-filled syringe vaccine, VIMKUNYA™ provides a crucial new tool to help protect at-risk individuals traveling to regions where the CHIKV is spreading."
"Chikungunya virus remains a significant global health concern. We're confident in the ability of VIMKUNYA™ to address this previously unmet medical need in adolescents as well as offer prevention for "adults."
According to Bavarian Nordic, access to VIMKUNYA™ depends on location and individual circumstances.
In some areas, a prescription may be required, so travelers should check with their healthcare provider or a travel health clinic to determine availability and whether they need a prescription. Patients may also ask their employer about coverage as a travel health expense if receiving VIMKUNYA™ for work-related travel.
Those visiting or living in areas where Chikungunya is present or with risk factors for severe disease should consult a healthcare professional to assess their need for vaccination.
"The age indication is significant as children tend to be less diligent in using insect repellants," Duellyn Pandis, DNP, MS, APRN, FNP-C, informed Vax-Before-Travel News.
"Also important is this vaccine can be used in immune-compromised individuals as it is not a live vaccine," added Pandis, President & CEO of Passport Health of Tampa Bay.
Vimkunya vaccination triggers the production of neutralizing antibodies at 22 days and up to 183 days post-vaccination. Studies have shown that VLP vaccines are highly immunogenic, have a proven safety record, and typically elicit high titer-neutralizing antibodies needed to protect against CHIKV.
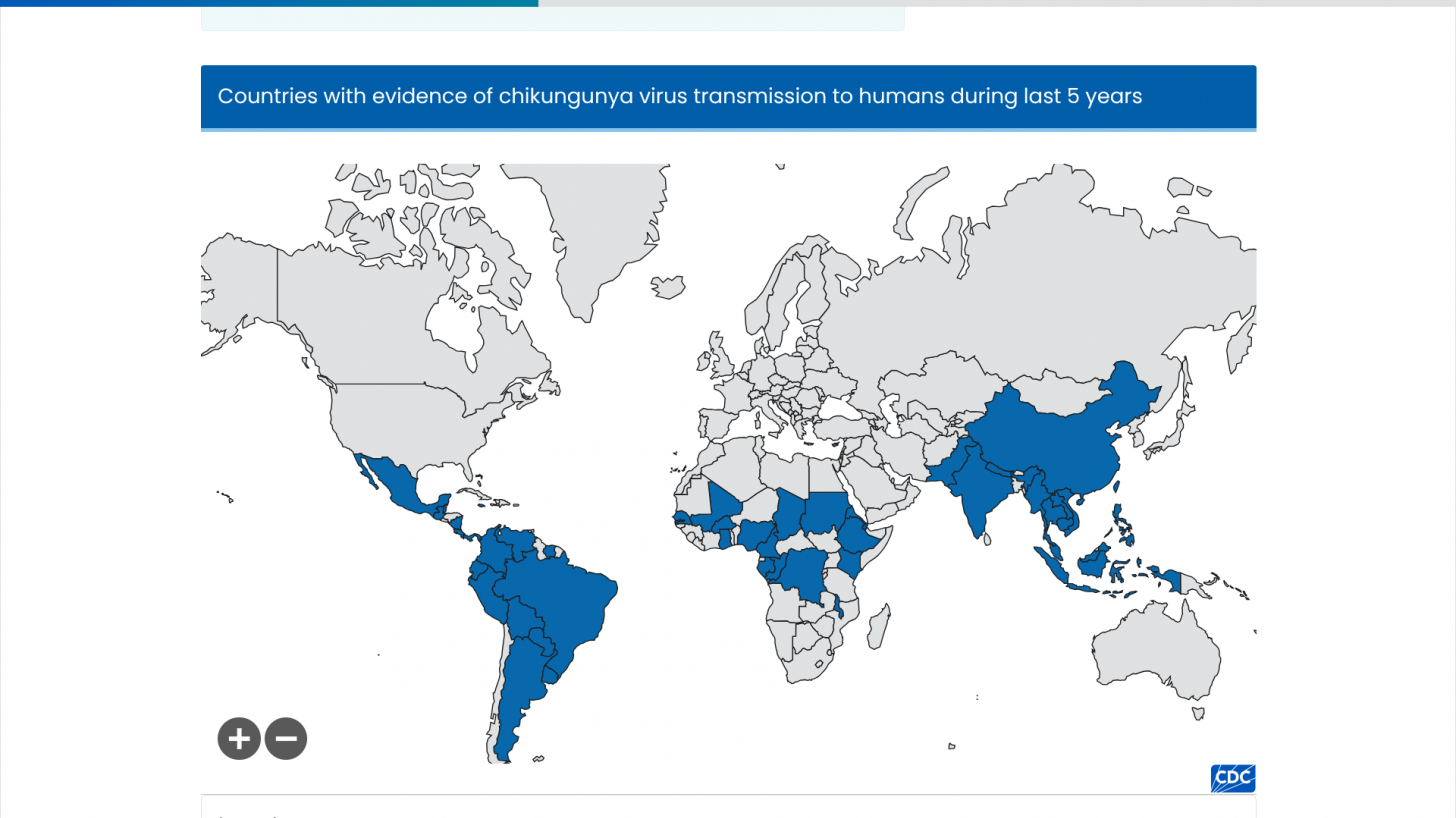
As of March 2025, the U.S. Centers for Disease Control and Prevention (CDC) lists countries and territories with evidence of CHIKV transmission. From 2006 to 2023, about 4,500 travel-related CHIKV cases were reported in the U.S. in areas such as California, Florida, Massachusetts, Puerto Rico, and Texas.
Additionally, the CDC Level 2 Advisory was reissued this year regarding the expanding Chikungunya outbreak in La Réunion island, an overseas department and region of France.
Since the beginning of 2025, 8,600 locally acquired cases have been reported in this vacation destination, which is located about 500 miles east of Madagascar.
The CDC recommends speaking to your healthcare provider about vaccination options if traveling to an area at risk for Chikungunya.
Note: This news article was updated with a travel vaccine expert insight on March 24, 2025.
Our Trust Standards: Medical Advisory Committee