Who Gets Lyme Disease
In the United States, recent studies suggest that approximately 476,000 people are diagnosed with Lyme disease (LD) each year. But who are these people?
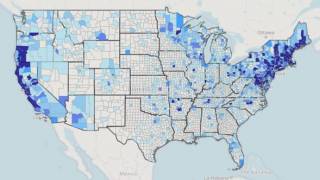
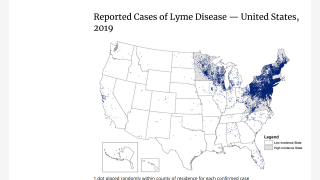
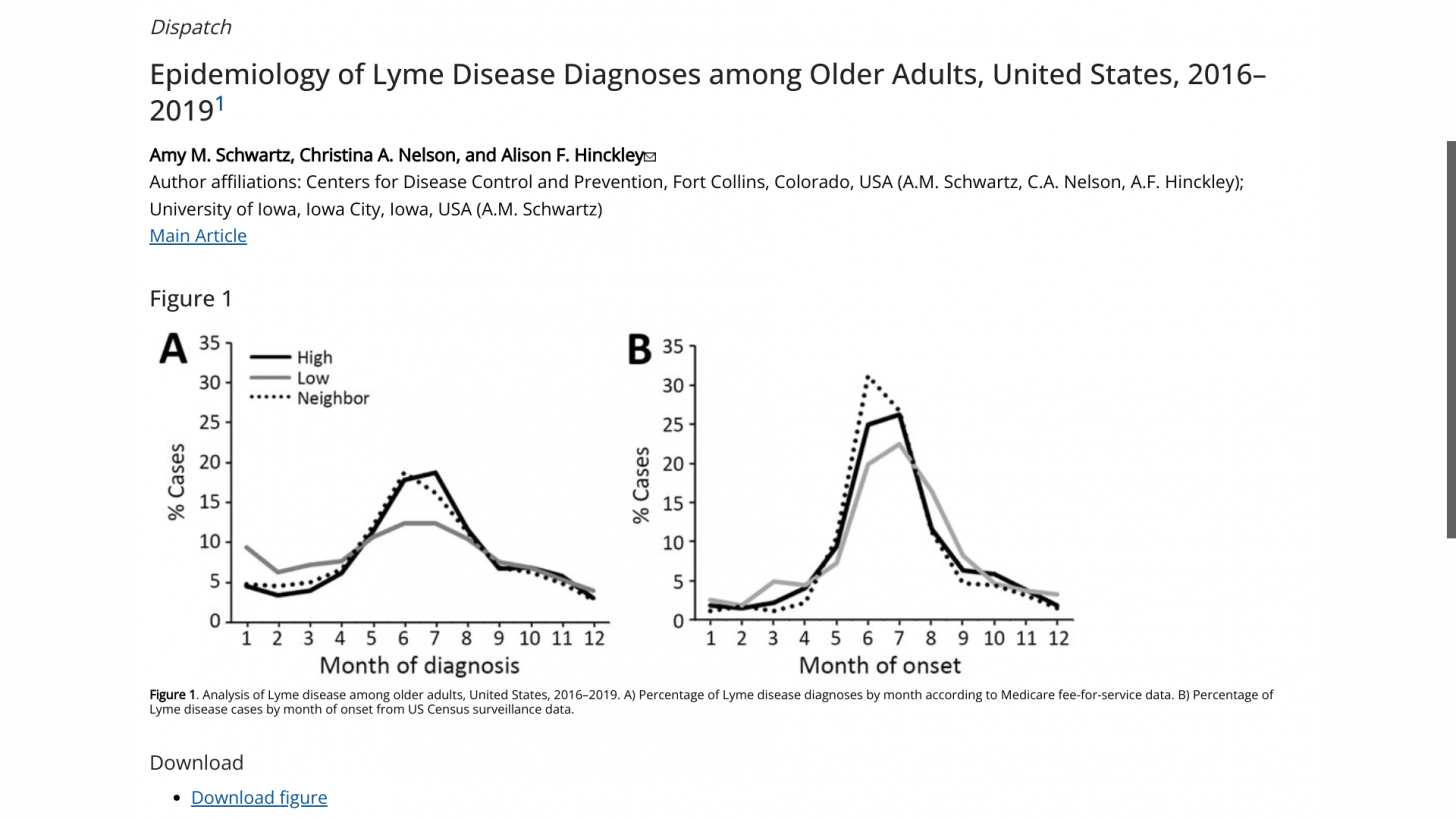
The U.S. Centers for Disease Control and Prevention published a study in Emerging Infectious Diseases indicating that most LD diagnoses in the United States occurred during the summer, primarily among older men and individuals living in high-incidence states.
Furthermore, on August 14, 2024, the CDC reported (Volume 30, Number 9—September 2024) that the overall incidence of LD was about seven times higher than that reported through public health surveillance.
About 82% of LD diagnoses occurred in high-incidence states by men aged 65–69 years.
A total of 56.1% of LD cases were diagnosed in men per Medicare data, while 60.4% in men were represented in surveillance data.
In surveillance data, women had a higher rate than men among those aged 75 to 79 in low incidence areas.
Lyme disease is caused by the bacterium Borrelia burgdorferi and, rarely, Borrelia mayonii. It is transmitted to humans through the bite of infected Ixodes ticks.
These new findings align with previous CDC reports.
These researchers wrote, 'When comparing Medicare and surveillance data, the seasonality of LD differed by region: high-incidence states and neighboring states exhibited similar patterns for diagnoses and surveillance cases, but low-incidence states demonstrated a more muted peak for LD diagnoses in summer.'
From a disease prevention perspective, there is good news on the horizon.
On July 17, 2024, Pfizer Inc. and Valneva SE announced that the “Vaccine Against Lyme for Outdoor Recreationists” clinical study had completed the primary vaccination series (three doses) of VLA15, a multivalent recombinant protein vaccine. It is designed for prophylactic, active immunization against LD to protect people against most human pathogenic Borrelia species.
Subject to positive data, VLA15 could be the first Lyme disease vaccine to become commercially available.
Following successful clinical research, Pfizer plans to submit a Biologics License Application to the U.S. FDA and a Marketing Authorization Application to the European Medicines Agency in 2026 for VLA15's authorization.
Our Trust Standards: Medical Advisory Committee