Antibody-Specific Lyme Disease Tests Announced

Two new Lyme IgG and Lyme IgM EIA kits for detection of antibodies against Lyme disease-causing bacteria were announced today.
The separate IgG and IgM assays provide laboratories the ability to offer more specificity in their Lyme screen algorithm.
The two assays give laboratories a more diverse arsenal of testing methodologies that enables them to achieve more reliable Lyme disease test results, stated California based Gold Standard Diagnostics Group (GSD).
They are designed for optimal functionality in diagnostic laboratories with a total incubation time of 45 minutes at room temperature with ready-to-use controls. The kits can be run manually or on any open automated EIA platform.
Furthermore, the GSD Lyme IgG and Lyme IgM EIA assays were made to provide antibody specific reliable information to the clinicians diagnosing this disease, said the company.
As of October 5, 2020, these assays are available in the USA.
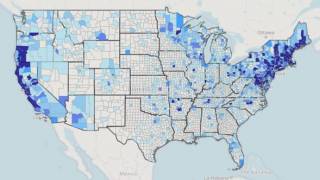
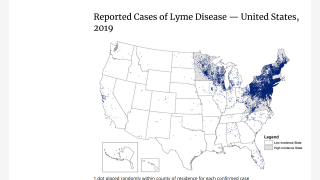
This is important news since ‘untreated Lyme disease can produce a wide range of symptoms, depending on the stage of infection’ says the US Centers for Disease Control and Prevention (CDC). Lyme disease is the most commonly occurring vector-borne disease and the 6th most commonly reported notifiable infectious disease in the USA.
“We are witness to major changes in Lyme diagnostics. Our team of professionals is adapting and responding to these changes,” states Dr. Barry Menefee, Infectious Disease Specialist at GSD.
Experts have recently found, Americans infected with Lyme disease is 10x higher than what is reported by the CDC.
A new study tracked the diagnostic testing of 5.2 million people throughout the USA and found that as many as 300,000 Americans contract Lyme disease each year.
This data substantially differs from 33,666 confirmed and probable cases of Lyme disease reported to the CDC in 2018, which was about 21 percent fewer cases than in 2017.
This under-diagnosing issue is related to Lyme disease symptom variability that it is often quite challenging to diagnose and can range from fever and headaches to irregular heartbeats, dizziness, and nerve pain.
The CDC says ‘Lyme disease patients treated with appropriate antibiotics in the early stages of Lyme disease usually recover rapidly and completely. Antibiotics commonly used for oral treatment include doxycycline, amoxicillin, or cefuroxime axetil.’
A specialty vaccine company based in France announced positive initial results for its first Phase 2 study of Lyme disease vaccine candidate VLA15.
In a press statement published on July 22, 2020, Valneva SE stated the VLA15 vaccine candidate was found immunogenic across all dose groups tested. And the VLA15-201 study’s results did not indicate that prior exposure to Lyme (seropositivity) has an impact on immunogenicity or safety.
VLA15 is an investigational multivalent protein subunit vaccine that targets the outer surface protein A (OspA) of Borrelia.
PrecisionVaccinations publishes Lyme disease vaccine and therapeutic news.
Our Trust Standards: Medical Advisory Committee