Stopping the Spread of Polio Requires Action
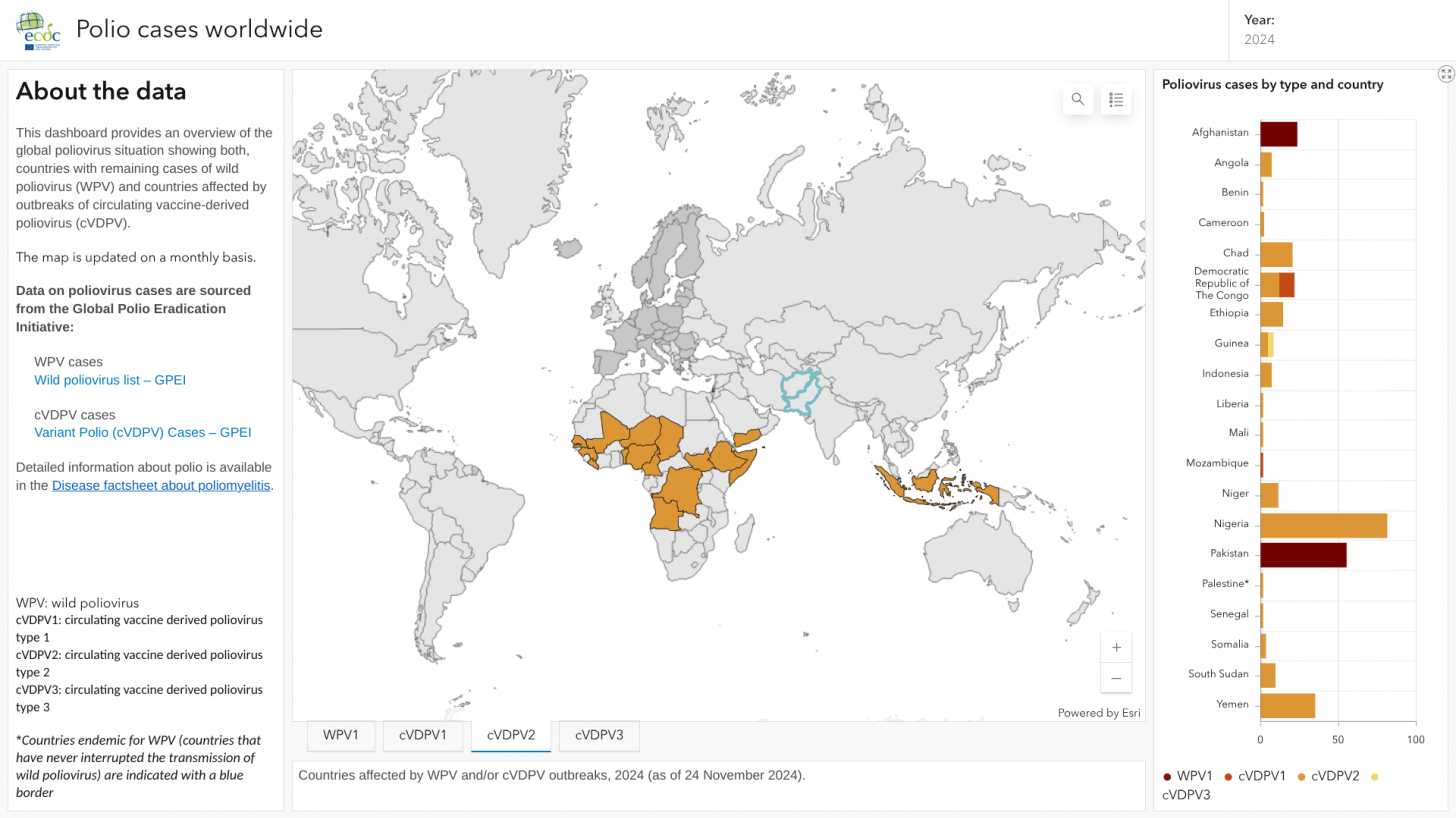
When the World Health Organization (WHO) confirmed at the end of 2024 that poliovirus detections and polio outbreaks remained a Public Health Emergency of International Concern, various agencies reviewed options to end this vaccine-preventable disease.
This WHO summary report from the Strategic Advisory Group of Experts on Immunization (SAGE) meeting on March 13, 2025, is now available for the general public's review.
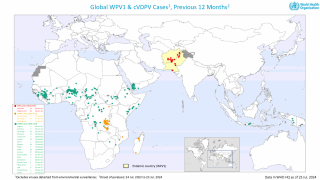
Regarding polio eradication, SAGE raised urgent concerns that, despite the alarming rise in wild poliovirus cases within endemic regions, decisive action toward transformative changes in the eradication strategy has been lacking.
Additionally, SAGE highlighted the threat posed by circulating variant polioviruses, which are expanding into new territories, including several European nations.
From September through December 2024, vaccine-derived poliovirus type 2 was detected in wastewater systems of 16 European cities.
SAGE reaffirmed its strong support for the safe cessation of bivalent oral poliovirus vaccine (OPV) and endorsed the planning of pre-cessation vaccination campaigns.
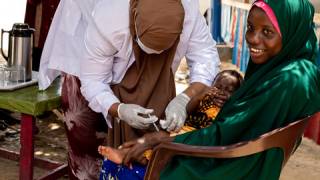
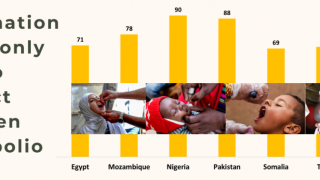
In February 2025, a mass polio vaccination campaign with novel oral polio vaccine type 2 (nOPV2) reached nearly 603,000 children. In recent years, this vaccine has been administered over one billion times.
Furthermore, SAGE endorsed a risk-grading framework to guide countries contemplating transitioning to IPV-only routine immunization schedules.
It has been determined that a three-dose schedule of inactivated poliovirus vaccine (IPV) and whole-cell pertussis-containing hexavalent vaccine starting at six weeks of age or later is adequate, without the need for a scheduled IPV booster dose (4th dose).
However, this SAGE recommendation does not change WHO's existing recommendations for booster doses of other antigens in the second year of life, namely diphtheria, tetanus, and pertussis-containing vaccines.
In conclusion, SAGE has revised its recommendations to reflect the latest evidence.
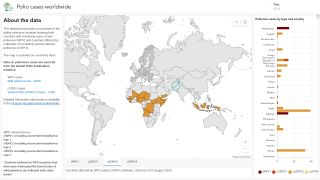
As of March 23, 2025, the Global Polio Eradication Initiative uses two types of vaccine to stop polio transmission: IPV and OPV.
In the United States, the IPV has been exclusively offered since 2000.
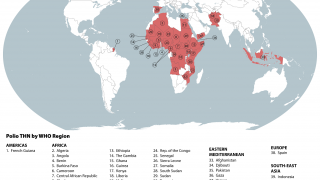
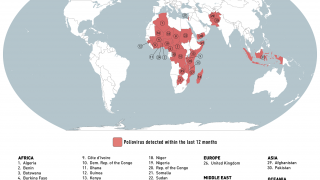
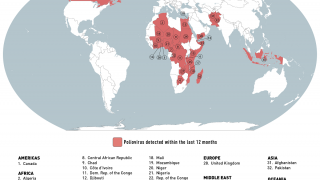
When traveling to one of the 39 identified polio-risk areas in March 2025, the U.S. CDC suggests speaking with a travel vaccine expert about immunization options.
Our Trust Standards: Medical Advisory Committee