Lyme Disease Vaccine Candidate Posts Positive Results

A France based specialty vaccine company announced positive initial results for its first Phase 2 study of Lyme disease vaccine candidate VLA15.
VLA15 is an investigational multivalent protein subunit vaccine that targets the outer surface protein A (OspA) of Borrelia.
In a press statement published on July 22, 2020, Valneva SE stated the VLA15 vaccine candidate was found immunogenic across all dose groups tested. And the VLA15-201 study’s results did not indicate that prior exposure to Lyme (seropositivity) has an impact on immunogenicity or safety.
And when compared to a previous Phase 1 study, the higher doses used in this trial elicited higher antibody responses across all serotypes.
Furthermore, in the age group comparable to the age group investigated in Phase 1 (18-49 years), the Seroconversion rates (SCR) ranged from 85.6% to 97%. The immunological response in older adults, one of the main target groups for a Lyme vaccine, is particularly encouraging, stated the company.
“We are pleased to report a successful first Phase 2 trial of our vaccine candidate against Lyme disease, a severe infection which affects an increasing number of people each year. Further data from the ongoing Phase 2 trials in the coming months will support further dose and schedule decisions, commented Wolfgang Bender, M.D., Ph.D., of Valneva.
“We are closely working with Pfizer to advance the development of VLA15 expeditiously.”
Valneva and Pfizer announced a collaboration for VLA15’s development and commercialization at the end of April 2020, and are working on the next development steps.
The medical need for vaccination against Lyme disease is steadily increasing as the disease footprint widens.
As part of further Phase 2 data to be released in a few months, an analysis of the functionality of the antibodies generated with VLA15 will be conducted. In close collaboration with regulatory authorities, Valneva has developed a Serum Bactericidal Antibody assay (“SBA”) for that purpose.
This first Phase 2 study, conducted in the EU and US, included 572 healthy adults aged 18 to 65 years.
In the main study phase, 452 subjects received one of two dose levels (either 135µg or 180µg) of VLA15 (approximately 180 subjects each) in three injections (Days 1, 29, and 57) or placebo (approximately 90 subjects).
Immunogenicity was measured by determining IgG antibodies against each of the six most prevalent Outer Surface Protein A serotypes of Lyme borreliosis in the US and Europe covered by the vaccine.
The endpoint readout was immunogenicity at Day 85 (one month after the finalization of primary immunization).
Valneva expects to report top-line results for the second Phase 2 study, VLA15-202, in a few months. In the VLA15-202 study, identical doses to the VLA15-201 study were tested using a longer vaccination schedule (Days 1, 57, and 180).
Lyme disease is a systemic infection caused by Borrelia bacteria transmitted to humans by infected Ixodes ticks. It is considered the most common vector-borne illness in the Northern Hemisphere. Left untreated, the disease can disseminate and cause more serious complications affecting the joints, the heart, or the nervous system.
>> Ulta Lab Lyme Disease Test <<
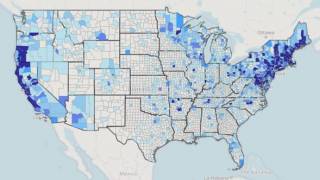
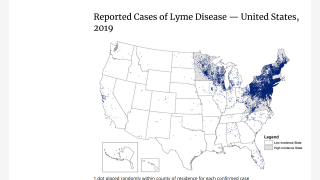
According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 300,000 Americans are diagnosed with Lyme disease each year (map), with at least a further 200,000 cases in Europe. Recently, the CDC estimated that there are approximately 10 times more people diagnosed with Lyme disease than the yearly reported number.
The history of Lyme disease began in 1975 when a cluster of children and adults residing in Lyme, Connecticut. By 1977, the first 51 cases of Lyme arthritis were described, and the Ixodes scapularis (black-legged) tick was linked to the transmission of the disease
In January 2020, a study reported reducing the Borrelia burgdorferi pathogen in this mouse population could reduce the number of human Lyme disease cases. About half of the infected Blacklegged ticks that spread Lyme disease in people initially become infected from white-footed mice.
Valneva SE has operations in Austria, Sweden, the United Kingdom, France, Canada, and the USA with over 500 employees.
Lyme disease vaccine development news is published by Precision Vaccinations.
Our Trust Standards: Medical Advisory Committee