Enhanced Lyme Disease and Tick-Borne Relapsing Fever Tests Announced

A California based tick-borne disease testing lab announced the availability of the Broad Coverage Ab (BCA) Assays for both Lyme disease and Tick-Borne Relapsing Fever (TBRF).
According to IGengeX’s press release on November 20, 2019, these BCA assays produce either a positive or negative result for exposure to Borreliae.
It is important to test for each Borreliosis disease because the TBRF Borreliae group can also cause Lyme-like symptoms, and the treatments may be different, says IGengeX.
The Lyme and TBRF BCA Assays are designed to detect antibodies for multiple species and strains of Lyme Borreliae and TBRF Borreliae, respectively, in human serum.
A positive test suggests exposure to the Lyme Borreliae group or the TBRF Borreliae group and should be used in conjunction with patient clinical symptoms and history.
For specific protein or band information, IgM and IgG ImmunoBlot tests should be ordered to provide more information on the stage of the disease and possible speciation of the Borrelia, says the company.
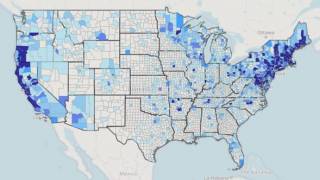
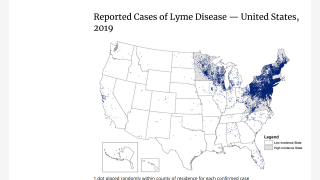
Recently, the Centers for Disease Control and Prevention (CDC) announced that Lyme disease is more common than previously thought, with over 400,000 cases diagnosed each year in the USA.
The CDC estimates suggest that Lyme disease may be underreported and that the true incidence is likely 10 times greater.
Lyme disease is transmitted to humans by a bite from a tick infected by the Borrelia burgdorferi bacteria,
And Lyme Disease is also an expensive medical burden.
According to a previous study, the U.S. health care system spends between $712 million and $1.3 billion a year ($3,000 per patient), related to Lyme disease treatments.
To address these issues, a new National Institutes of Health (NIH) Strategic Plan for Tickborne Disease Research was published on October 9, 2019, which focuses on the scientific priorities over the next 5 years.
This innovative NIH plan prioritizes the research needed to prevent tick-borne diseases. At least 20 different disease-causing bacteria, viruses, and parasites are known to transmit ticks to people, such as Lyme Disease.
Lyme disease news
- October 12th, 2019 – Single multiplexed assays could replace the standard 2-tiered algorithm recommended for the laboratory diagnosis of Lyme disease, reported a team at Columbia University Engineering.
- September 30th, 2019 – French biotech company Valneva SE announced that it has completed patient recruitment of the Phase 2 studies for its Lyme disease vaccine candidate, VLA15. This is good news since VLA15 is currently the only active vaccine program in clinical development against Lyme disease. The program was granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in July 2017.
Dr. Jyotsna Shah, President and Laboratory Director of IGeneX, said in a press release, "Lyme and TBRF are continuing to spread, and sick patients from all walks of life need to be able to get an accurate diagnosis so they can begin the appropriate treatment."
Advantages of the Broad Coverage Ab Assays for Lyme disease and TBRF:
- Detect both IgM and IgG antibodies
- Far broader and more inclusive of Borrelia species than standard serologies
- Detects antibodies to Borrelia species and strains from North America, Europe, and Australia
- Better than two-tier ELISA/Western Blot and two-tier ELISA
- Offers simple, cost-effective and easy-to-understand positive or negative results.
Each Lyme or TBRF BCA assay costs $195 dollars and is available through a healthcare provider. Visit IGeneX for more information.
Previously, the CDC issued new recommendations for a 2-tier test for Lyme disease diagnosis.
On August 15, 2019, the CDC said ‘when cleared by the FDA, serologic assays that utilize enzyme immunoassay rather than western immunoblot assay used in a 2-test format, are acceptable alternatives for the laboratory diagnosis of Lyme disease.’
Additionally, ‘clinicians and laboratories should consider serologic tests cleared by the FDA as CDC-recommended procedures for Lyme disease serodiagnosis.’
Lyme disease vaccine news published by Precision Vaccinations
Our Trust Standards: Medical Advisory Committee