Lyme Disease Vaccine Needed in Both Europe and the USA
While most hikers and campers await the results of a Phase 3 clinical trial of the most advanced Lyme disease vaccine candidate, The Lancet Infectious Diseases recently published an article highlighting very positive data from the Phase 2 study.
On April 25, 2025, results from the NCT04801420 study confirm the previously observed safety and immunogenicity profiles of VLA15 in adults and extend them to children aged five years and older, as well as adolescents.
Additionally, the greater immunogenicity of VLA15 among children and adolescents might translate to increased flexibility in the real-world clinical setting.
According to Vaneva SE and Pfizer Inc., VLA15 is an investigational multivalent protein subunit vaccine that utilizes an established mechanism of action for a Lyme disease vaccine, targeting the outer surface protein A (OspA) of Borrelia burgdorferi, the bacterium that causes Lyme disease.
OspA is a surface protein expressed by the bacteria when present in a tick. Blocking OspA inhibits the bacterium’s ability to leave the tick and infect humans.
The vaccine candidate covers the six most prevalent OspA serotypes expressed by the Borrelia burgdorferi sensu lato species in North America and Europe.
Lyme disease, if left untreated, can cause serious chronic complications affecting the skin, joints, heart, or nervous system.
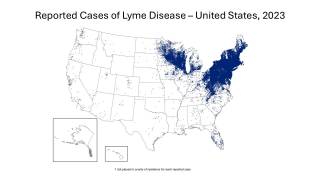
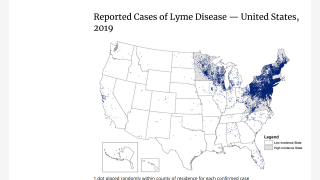
According to various health agencies, the medical need for vaccination against Lyme disease is steadily increasing as the geographic footprint of the disease widens in the United States and Europe.
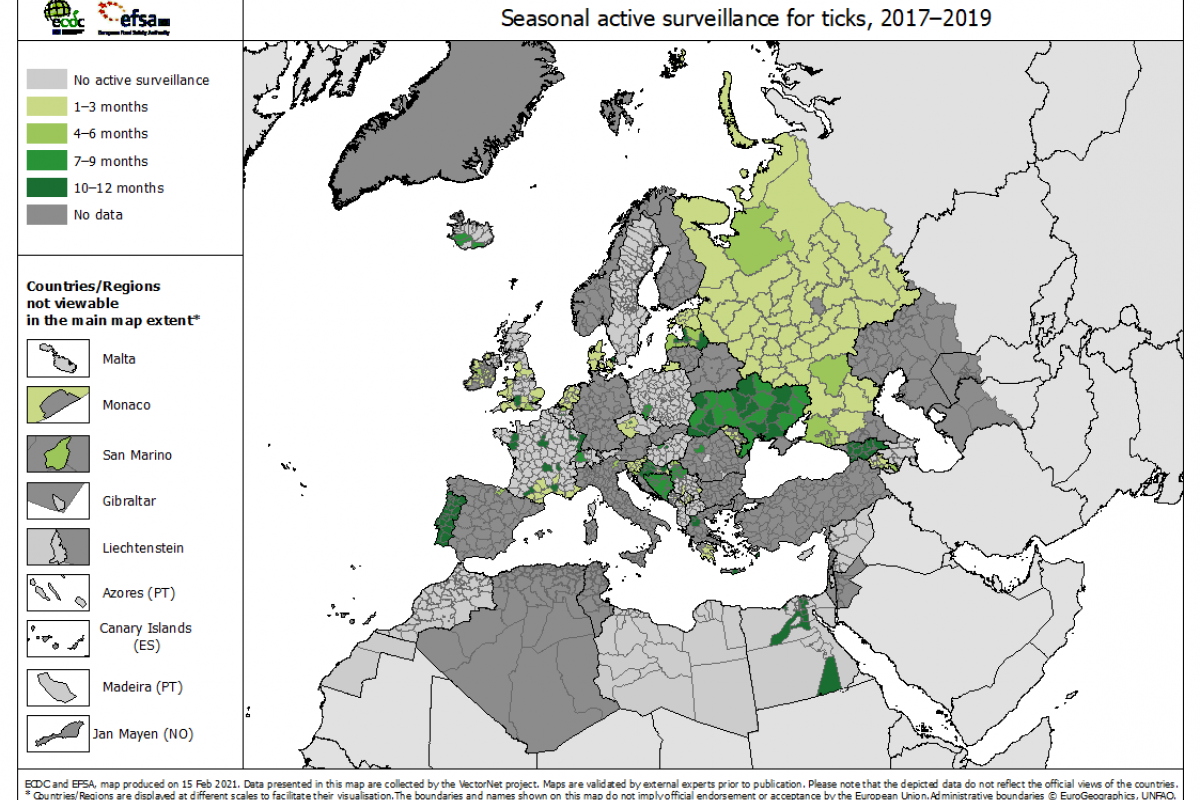
Throughout Europe, the bacteria are only transmitted by the bites of the ticks Ixodes ricinus and I. persulcatus. In the most affected regions, tick infection rates may exceed 10%. These areas are primarily located in central Europe.
However, the ECDC says in recent years, the spread of infected ticks has extended toward northern latitudes, including Scandinavia.
In the United Kingdom, the South of England and the Scottish Highlands have been earmarked by the government as high-risk areas for Lyme disease in 2025. Annually, the UK Health Security Agency reports about 1,500 laboratory-confirmed cases of Lyme disease.
With millions of people returning to the woods and mountains this summer, analysts say there is significant pent-up demand for an effective Lyme disease vaccine.
Our Trust Standards: Medical Advisory Committee