Lyme Disease Vaccine Coming Soon

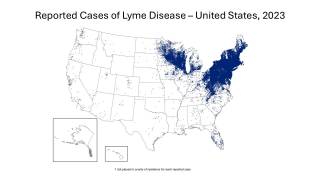
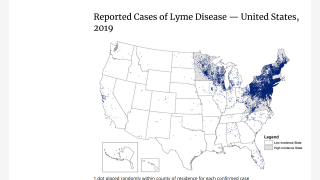
Without an approved Lyme disease vaccine available, many people who enjoy the outdoors have concerns about planning hikes for Spring 2025. According to the World Health Organization, Lyme disease cases are common and expanding in Europe, the United Kingdom, and the United States.
But there is hope on the horizon.
Vaneva SE announced on February 18, 2025, that the first data readout for the Lyme disease vaccine candidate (VLA15) phase 3 is expected by the end of 2025.
This indicates that regulatory agencies may consider authorization in 2026. Valneva'shler, Valneva's Chief Financial Officer, commented in a press release, "Once again, we successfully delivered double-digit sales growth ...We made significant clinical and regulatory progress last year, setting the stage for several important catalysts to drive value in 2025, most notably with the first Phase 3 study results for our lead Lyme disease vaccine candidate, VLA15."
VLA15 is a multivalent recombinant protein vacBorrelia'sting Borrelia's outer surface protein A (OspA). It is designed for protective, active immunization against most human pathogenic Borrelia species. OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick bite.
A study published in late 2024 determined that 50% of adult blacklegged ticks carry the bacteria that causes Lyme disease, while up to 25% of the younger (nymph) blacklegged ticks carry the bacteria.
Until a preventive vaccine becomes commercially available, avoiding tick bites is the best way to prevent Lyme disease.
Our Trust Standards: Medical Advisory Committee