Will Millions of Monkeypox Vaccines be Needed?

The U.S. Department of Health and Human Services (HHS) announced yesterday that it is making up to 442,000 doses of the Jynneos® vaccine available under an accelerated Phase of the National Vaccine Strategy (NVS) to combat the current monkeypox virus (MPXV) outbreak in the USA.
In light of the recent U.S. FDA Emergency Use Authorization and revised dosing guidelines from the Centers for Disease Control and Prevention, HHS doubled the number of doses available as initially anticipated.
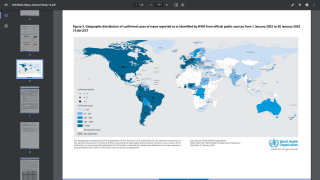
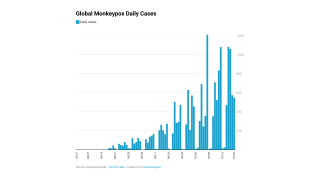
These Jynneos vaccines are certainly needed as the number of MPXV cases has doubled over the past few weeks, reaching about 12,000 cases as of August 16, 2022.
"We are pleased to make these additional doses available to states and jurisdictions faster than originally anticipated," commented HHS Assistant Secretary for Preparedness and Response Dawn O'Connell in a press release issued on August 15, 2022.
"We continue to pursue all options that allow us to increase the number of vaccine doses available, expedite their delivery to places that need them, and strengthen the vaccine supply chain."
In addition to accelerating access to doses under Phase 3, this HHS action paves the way for launching Phase 4 of the NVS later in August, also faster than anticipated.
However, the NVS may need to be expanded based on new research from France.
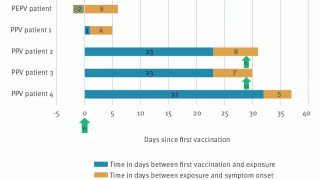
The peer-review Annals of Internal Medicine published findings from a study from Bichat-Claude Bernard Hospital in Paris that conducted monkeypox tests on anorectal swabs.
This report documents positive MPXV PCR results from anal samples in asymptomatic MSM.
Also, of the 187 asymptomatic participants who tested negative for MPXV, three presented at the clinic more than three weeks after the initial MPXV-negative anal swab with symptoms and tested positive.
The study authors commented that 'it's unclear if viral shedding can lead to MPXV transmission.'
'If so, postexposure ring vaccination around people with probable or confirmed MPXV infections might not be enough to contain the outbreak.'
In reaction to this finding, French recommendations began advising Jynneos (MVA-BN, IMVANEX®, IMVAMUNE®) vaccination for all MSM with multiple partners in mid-July 2022.
If the U.S. government adopts France's vaccination policy, it may need millions of additional vaccines.
Currently, the standard U.S. FDA regimen for Jynneos involves a subcutaneous route of administration with an injection volume of 0.5mL.
And the authorized alternative regimen involves an intradermal route of administration with an injection volume of 0.1mL.
The second dose of Jynneos should be given 28 days after the first dose.
PrecisionVaccinations publishes fact-checked, research-based vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee