Europe Extends Locally Produced Monkeypox Vaccine Authorization

Denmark-based Bavarian Nordic A/S recently announced that the European Commission (EC) extended the marketing authorization for the Imvanex® smallpox vaccine to include protection from monkeypox and disease caused by the vaccinia virus.
The new EC approval is valid in all European Union Member States as well as in Iceland, Liechtenstein, and Norway.
‘The monkeypox indication approval is an example of great cooperation between Bavarian Nordic and the European regulators as such an indication extension normally takes at least 6-9 months to achieve, 'wrote the company.
Paul Chaplin, President and CEO of Bavarian Nordic, commented in a press release issued on July 25, 2022, “The availability of an approved vaccine can significantly improve nations’ readiness to fight emerging diseases, but only through investments and structured planning of the biological preparedness.”
“The development of Imvanex was made possible through significant investments from the U.S. government for the past two decades, leading the way for other governments to develop plans and prioritize for the future to protect their citizens against public health threats.”
IMVANEX® (MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic) is a non-replicating smallpox vaccine approved by the EC in 2013 for immunization against smallpox in adults aged 18 years and older and has subsequently gained regulatory approvals in Canada (marketed as IMVAMUNE®) and the U.S. (marketed as JYNNEOS®) where the approvals have been extended to include the monkeypox indication.
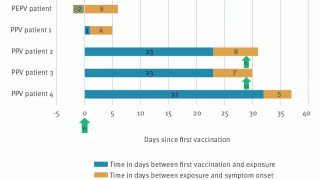
In recent years, smaller quantities of the vaccine have been supplied in response to sporadic cases of monkeypox.
Bavarian Nordic has ongoing supply contracts with USA and Canada and has delivered the vaccine to a number of undisclosed countries globally as part of their national biological preparedness.
As of July 21, 2022, the U.S. federal government plans to access more than 6.9 million doses by mid-2023.
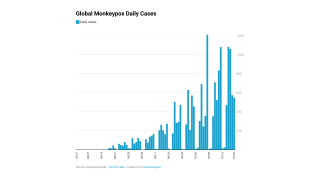
During the ongoing 2022 monkeypox outbreak, Bavarian Nordic has worked with several governments to fulfill the immediate demand for the vaccine through a number of supply agreements and is working to secure the manufacturing of vaccines to meet future demand.
This vaccine was developed to support immunocompromised individuals who are not recommended to receive traditional replicating smallpox vaccines.
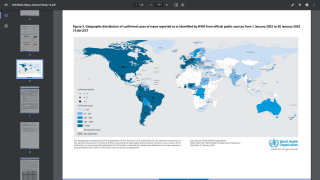
Recently, the WHO’s Director-General declared the ongoing monkeypox outbreak met his definition of a global concern and encouraged prevention measures, such as vaccinations.
Additional monkeypox outbreak news is posted at Vax-Before-Travel.com/Monkeypox.
Vax-Before-Travel publishes fact-checked, research-based travel vaccine news curated for international travelers.
Our Trust Standards: Medical Advisory Committee