Post Monkeypox Vaccination Breakthrough Infections Confirmed

Throughout the monkeypox outbreak, numerous countries and communities have sought Bavarian Nordic's JYNNEOS® vaccine to protect people from the fatal disease.
With millions of JYNNEOS doses delivered since May 2022, efficiency questions have remained unanswered.
Unfortunately, a new Research Letter published on September 30, 2022, indicates that the JYNNEOS vaccine does not deliver 100% protection.
Published by the peer-review journal The JAMA Network, these researchers describe monkeypox virus (MPXV) breakthrough infections after receiving the JYNNEOS (MVA, IMVANEX®, MVAMUNE®) vaccine at a Howard Brown Health location in Chicago, Illinois.
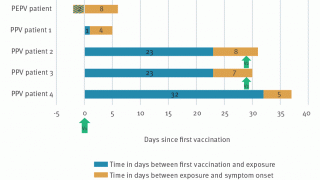
Eight monkeypox cases occurred more than 28 days from the first dose of JYNNEOS, of which two cases occurred more than 14 days from the second dose.
Because the incubation period for monkeypox is 3 to 17 days, some of the cases occurring between 1 and 14 days after JYNNEOS vaccination may not represent actual vaccine failure because patients may have sought vaccination after realizing they were exposed, wrote these researchers.
And more extensive clinical studies are needed to better understand vaccine effectiveness and durability of immune response with JYNNEOS against MPXV, concluded these researchers.
These researchers did not report any industry conflicts of interest, and the Corresponding Author is Aniruddha Hazra, MD, Howard Brown Health (anuh@howardbrown.org).
A previous non-peer-reviewed study published in August 2022 found similar breakthrough infections.
Ten out of 12 patients in France developed an MPXV infection in the five days following vaccination, and two had a breakthrough infection at 22 and 25 days.
Separately, the U.S. CDC Morbidity and Mortality Weekly Report published on September 30, 2022, suggests that a single JYNNEOS dose provides some protection against monkeypox infection.
Confirmed MPXV incidence was 14 times as high among unvaccinated males compared with those who had received a first JYNNEOS vaccine dose ≥14 days earlier.
The U.S. FDA approved the 2-dose JYNNEOS to prevent monkeypox disease in individuals at high risk for MPXV infection on September 24, 2019. FDA leaders indicate the best vaccine efficacy should be attained following the second dose.
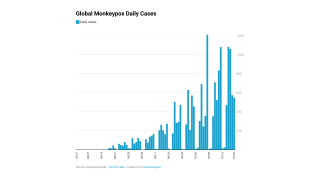
As of September 30, 2022, the CDC confirmed 28 fatalities from over 100 reporting countries related to the monkeypox outbreak that began in May 2022.
Globally, there have been over 68,000 cases reported, with about 26,000 in the U.S.
Given the unknown effectiveness of vaccination in this MPXV outbreak, vaccinated people should continue to protect themselves from infection by avoiding close, skin-to-skin contact, including intimate contact, with someone who has monkeypox, says the CDC.
Additional MPXV outbreak news is posted at Monkeypox Today.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee
- Human Monkeypox Virus Infection in the Immediate Period After Receiving Modified Vaccinia Ankara Vaccine
- CDC JYNNEOS Vaccine
- Incidence of Monkeypox Among Unvaccinated Persons
- CDC MPXV Outbreak Cases and Data
- FDA approves smallpox and monkeypox vaccine
- Breakthrough infections after post-exposure vaccination against Monkeypox
- Monkeypox vaccines