Second Mpox Vaccination Still Recommended

In the U.S. Centers for Disease Control and Prevention (CDC) mpox vaccination analysis published today, all eligible persons are recommended to complete the two-dose JYNNEOS® vaccination series to optimize protection from infection.
JYNNEOS is a U.S. Food and Drug Administration (FDA) approved vaccine for the prevention of mpox and smallpox.
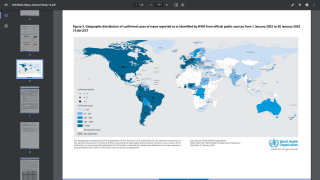
The CDC published the Morbidity and Mortality Weekly Report on December 30, 2022, which disclosed the protection of 276 mpox cases in persons who received one dose of JYNNEOS vaccine ≥14 days before illness onset and 6,329 cases in unvaccinated persons during the 2022 U.S. outbreak.
The percentage of hospitalized vaccinated patients (2%) was lower than that among unvaccinated patients (8%).
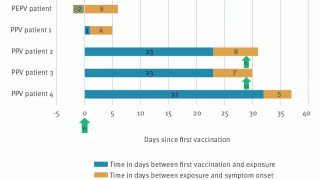
Vaccinated patients reported signs and symptoms similar to those described earlier in the outbreak. However, some symptoms were reported less frequently among vaccinated than among unvaccinated mpox patients.
And the odds of systemic signs and symptoms, such as fever and chills, were lower among vaccinated patients.
Furthermore, the frequent presentation of rash in the genital and perianal areas among both vaccinated and unvaccinated mpox patients suggests that sexual transmission in this population was a common mechanism of transmission.
The fewer number of reported rash locations among vaccinated patients suggests possible prevention of the spread of rash from the site of injection among even partially vaccinated persons.
The findings in this CDC report are subject to limitations, such as 47% of cases were excluded because of missing vaccination information; therefore, results might not be generalizable to all persons with mpox in the U.S.
And clinical data were not available for all cases.
In particular, HIV status was unknown or missing for two-thirds of vaccinated and more than one-half of unvaccinated patients.
Because HIV infection might affect the trajectory and illness severity, future studies with additional data for real-world use of the JYNNEOS vaccine against clinical outcomes, including data on HIV history, are needed.
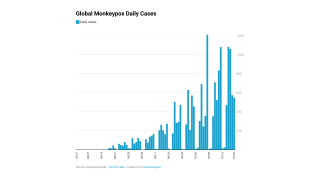
As of December 20, 2022, 1,152,073 JYNNEOS vaccine doses had been administered in 57 U.S. Jurisdictions since May 2022.
As of December 22, 2022, the CDC says there is limited clinical trial data on administering the Jynneos vaccine simultaneously with other vaccines, such as COVID-19.
The safety and immunogenicity of JYNNEOS have been established in people living with HIV, stated the CDC on August 5, 2022. And the UK NHS says the vaccine can be safely given to people who are living with HIV infection and those who are taking pre-exposure prophylaxis.
In addition, mpox vaccine news is posted at MpoxToday.
Disclosures: The CDC researchers who participated in this report did not disclose any industry conflicts of interest.
Our Trust Standards: Medical Advisory Committee