Monkeypox Intradermal Vaccination Offers Several Benefits
Despite limited clinical evidence, all available data suggest that intradermal administration of the Bavarian Nordic-produced Jynneos® vaccine will be as immunogenic as subcutaneous dosing for preventing monkeypox infection and illness, which leads us to favor intradermal use from both the individual and public health perspectives, wrote a group of vaccine experts.
'Vaccines work only if they are accessible, trusted, and used.'
The New England Journal of Medicine published a Perspective on September 29, 2022, written by John T. Brooks, M.D., Peter Marks, M.D., Ph.D., Robert H. Goldstein, M.D., Ph.D., and Rochelle P. Walensky, M.D., M.P.H., the director of the U.S. CDC, that explained the benefits of offering Jynneos intradermal vaccinations during the monkeypox outbreak in the USA.
Intradermal administration at one-fifth the standard dose is an attractive option that could ameliorate current supply shortages while ensuring similar levels of immunogenicity.
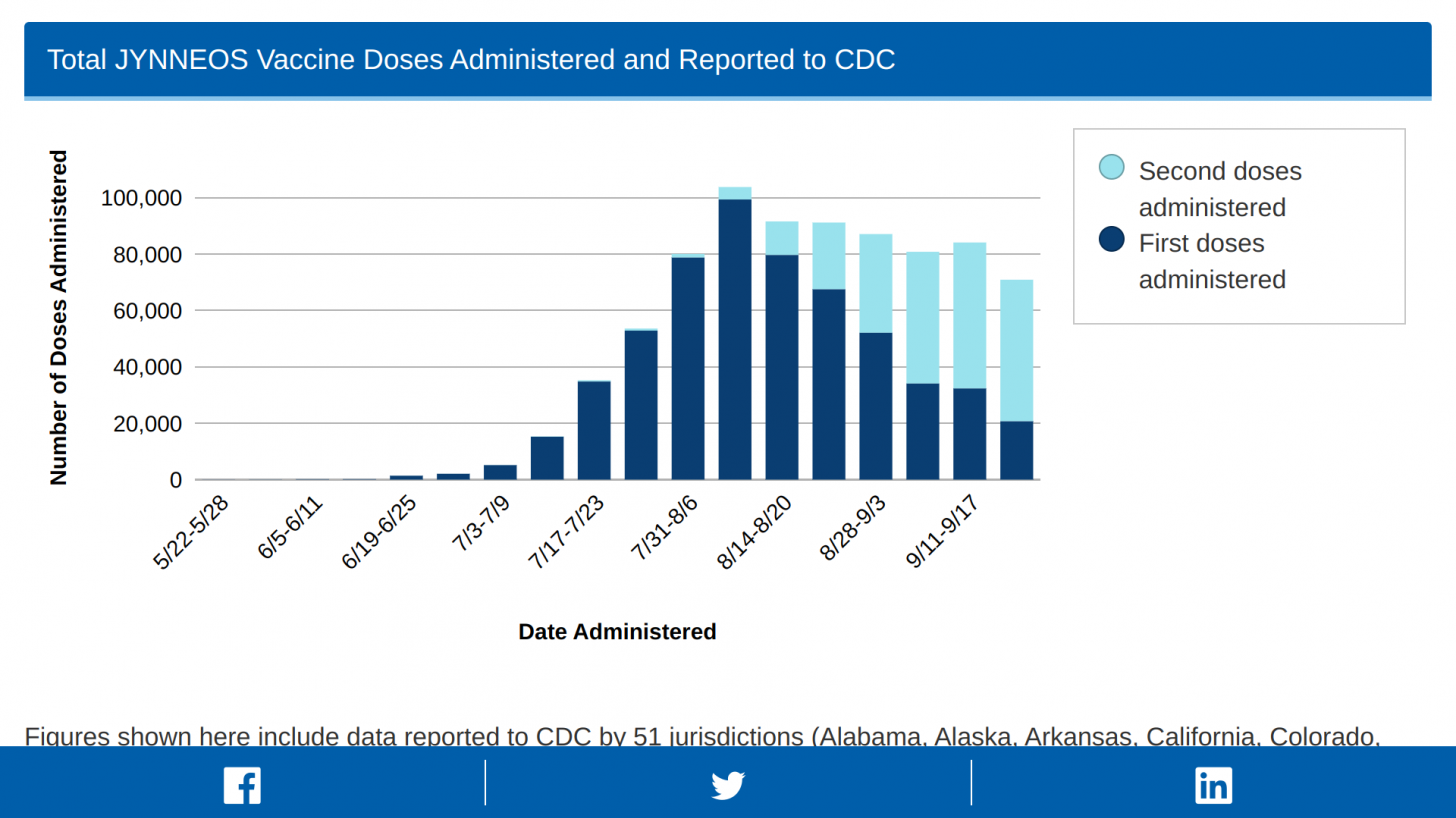
As of September 27, 2022, the U.S. CDC reported that 803,596 Jynneos doses had been administered in the 51 U.S. Jurisdictions reporting data.
This reflects a very efficient delivery of the 833,293 doses distributed by the U.S. HHS's Administration for Strategic Preparedness and Response on September 28, 2022.
This Perspectve is excerpted below:
Jynneos, sold internationally as Imvamune and Imvanex, contains a live but attenuated nonreplicating MVA strain and is licensed by the U.S. FDA to prevent both smallpox and monkeypox.
Among the advantages of intradermal vaccination is that it can generate immune responses equivalent to those achieved with subcutaneously or intramuscularly administered vaccine but with as little as 20% to 10% of the dose while avoiding the rare risk of nerve, blood-vessel, or joint-space injury.
In a randomized, controlled trial, a one-fifth dose of intradermally achieved neutralizing antibody levels similar to those produced with standard amounts given subcutaneously on the same FDA-approved schedule. However, by some measures, cellular immunity levels were lower.
A dose-finding study using a different but closely related MVA vaccine showed that even a one-tenth dose of MVA given intradermally was as immunogenic as the full dose given subcutaneously or intramuscularly.
This type of vaccination has been extensively studied to prevent a wide range of viral diseases, including influenza, Japanese encephalitis, hepatitis A, hepatitis B, HPV, polio, rabies, varicella zoster, and yellow fever.
However, there are no data from previous clinical trials explicitly evaluating the effectiveness of Jynneos against monkeypox using either route of administration, says the CDC.
"We don't know what level of immune response in someone given the vaccine as pre- or post-exposure prophylaxis would correlate with functional immunity against monkeypox infection or protection against severe disease," wrote these vaccine experts.
"But there are no a priori reasons to doubt that Jynneos will be effective by the intradermal route, and we have reasonable evidence that intradermal dosing will be similarly immunizing as compared with subcutaneous dosing, at least in the short term, as a means of outbreak control to break chains of transmission."
"In the meantime, we urge people at the highest risk for infection to receive both doses of the two-dose vaccine."
"We encourage manufacturers to consider routinely testing intradermal dose administration in future clinical vaccine trials to expand our understanding of this operationally attractive option."
"The currently available evidence suggests that shifting to intradermal dosing that requires less vaccine is not a lesser option."
"Rather, it is a rational, evidence-informed means of advancing access, equity, and our chances of controlling the monkeypox outbreak."
Note: The ECDC reported on September 28, 2022, monkeypox cases have declined since July 2022, but the likelihood of the disease spreading remains high. And the earliest date of symptom onset was reported as April 17, 2022.
Additional monkeypox outbreak news is posted at Monkeypox Today.
PrecisionVaccinations publishes fact-checked, research-based vaccine news manually curated for mobile readership.
Our Trust Standards: Medical Advisory Committee