The US Procures Ebola Treatment For National Preparedness
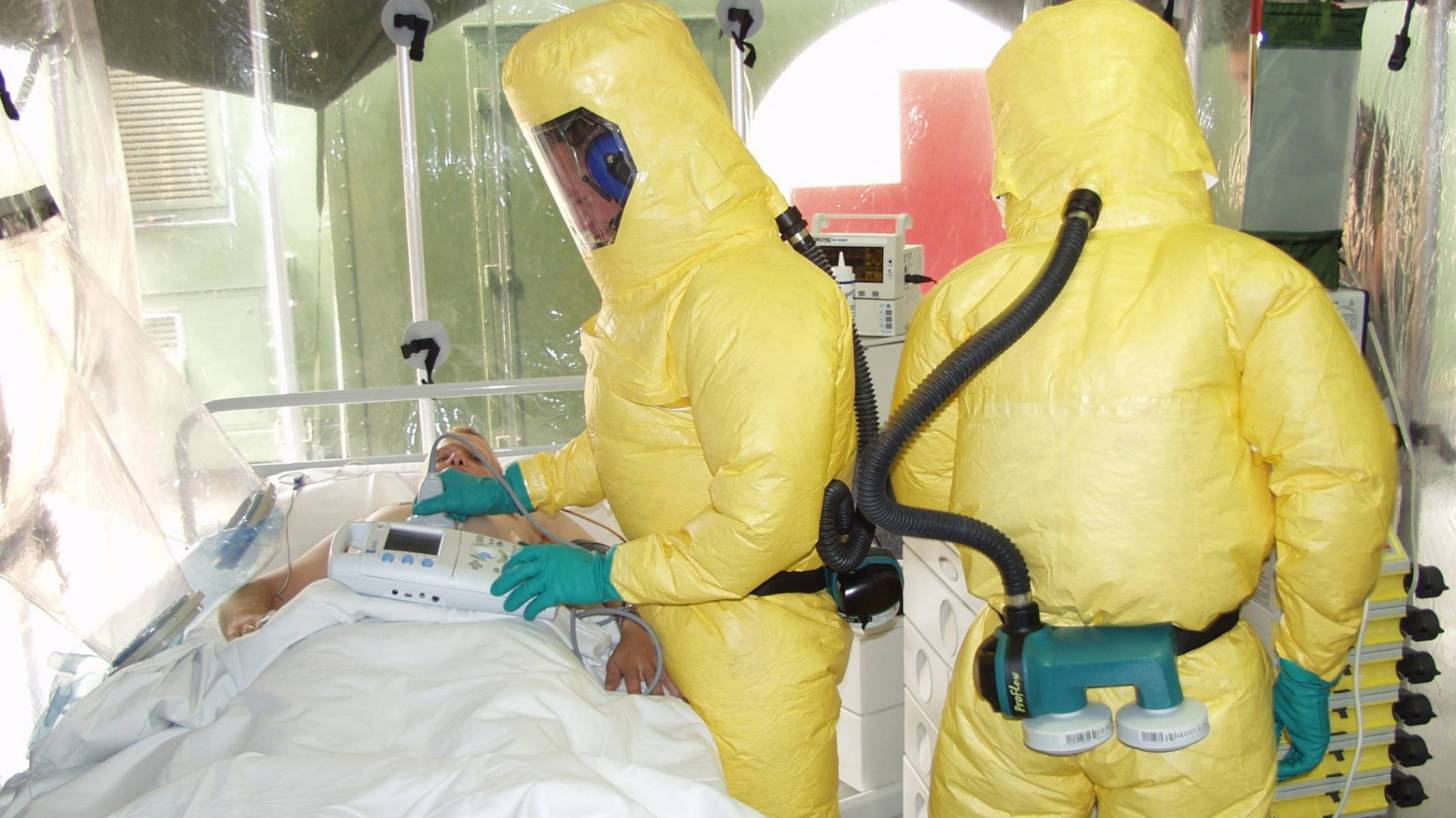
The Biomedical Advanced Research and Development Authority (BARDA) announced it entered an agreement to procure REGN-EB3 as part of the US government’s goal of building national preparedness for public health emergencies.
REGN-EB3 is New York-based Regeneron Pharmaceuticals, Inc. investigational triple antibody cocktail treatment for Ebola virus infection and is currently under Priority Review by the U.S. Food and Drug Administration (FDA), with a target action date of October 25, 2020.
Contingent on FDA approval, Regeneron said on July 29, 2020, expects to deliver to BARDA, which is part of the Office of the Assistant Secretary for Preparedness and Response within the U.S. Department of Health and Human Services (HHS), an established number of treatment doses over the course of 6-years.
In 2019, the randomized controlled PALM clinical trial conducted in the Democratic Republic of the Congo was stopped early when preliminary results showed that REGN-EB3 crossed the pre-specified superiority threshold for preventing death compared to the control arm, ZMapp®.
REGN-EB3 demonstrated superior efficacy compared to ZMapp across multiple measures, including reduced mortality and fewer days until the Ebola virus was no longer detected in the bloodstream.
During the trial, there were 3 serious adverse events for REGN-EB3, compared to seven for ZMapp.
"The current COVID-19 pandemic provides an important lesson in preparation for potential biological threats to our nation's health security," said BARDA acting director Gary Disbrow, Ph.D., in a press statement.
"Whether the next one is another coronavirus, an Ebola virus, or a completely novel disease, we must do everything we can to be prepared."
REGN-EB3 is being developed with collaboration and funding provided by BARDA under ongoing USG Contract Nos. HHSO100201700016C and HHSO100201500013C.
Regeneron is a biotechnology company that invents life-transforming medicines for people with serious diseases.
Updated Ebola vaccine development news and availability can be found at this link.
Precision Vaccinations publishes Ebola treatment news.
Our Trust Standards: Medical Advisory Committee