Ebola Treatment Approved by the U.S. FDA

The U.S. Food and Drug Administration (FDA) announced the approval of a mixture of three monoclonal antibodies called Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn), as the first FDA-approved treatment for Zaire ebolavirus (Ebola virus) infection in adult and pediatric patients.
Regeneron Pharmaceuticals’s Inmazeb targets the glycoprotein that is on the surface of the Ebola virus. Glycoprotein attaches to the cell receptor and fuses the viral and host cell membranes allowing the virus to enter the cell.
The three antibodies that comprise Inmazeb can bind to this glycoprotein simultaneously and block attachment and entry of the virus, stated the FDA press release on October 14, 2020.
“Today’s action demonstrates the FDA’s ongoing commitment to responding to public health threats—both domestically and abroad—on the basis of science and data,” added FDA Commissioner Stephen M. Hahn, M.D.
“This approval was made possible because of our steadfast dedication to facilitate the development of safe and effective treatments for infectious diseases as part of our vital public health mission.”
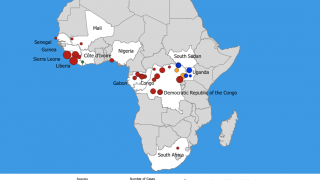
This FDA announced coincides with the ongoing 11th Ebola outbreak in Africa.
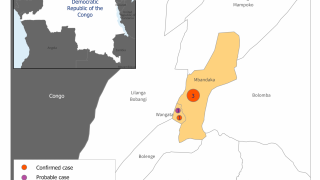
On June 1, 2020, the government of the Democratic Republic of the Congo (DRC) announced that a new outbreak of Ebola virus disease is occurring in the Wangata health zone, Mbandaka, in Équateur province.
Zaire ebolavirus, commonly known as Ebola virus, is one of (4) Ebolavirus species that can cause a potentially fatal human disease, says the FDA.
Ebola virus is transmitted through direct contact with blood, body fluids, and tissues of infected people or wild animals, as well as with surfaces and materials, such as bedding and clothing, contaminated with these fluids. Individuals who provide care for people with Ebola virus, including health care workers who do not use correct infection control precautions, are at the highest risk for infection.
Inmazeb was evaluated in 382 adult and pediatric patients with confirmed Zaire ebolavirus infection in one clinical trial (the PALM trial) and as part of an expanded access program conducted in the Democratic Republic of the Congo (DRC) during an Ebola virus outbreak in 2018-2019.
The PALM trial was led by the U.S. National Institutes of Health and the DRC’s Institut National de Recherche Biomédicale with contributions from several other international organizations and agencies.
“Today’s approval highlights the importance of international collaboration in the fight against Ebola virus,” stated John Farley, M.D., MPH, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research.
“The urgent need for advanced therapies to combat this infectious disease is clear, and today’s action is a significant step forward in that effort.”
In the PALM trial, the safety and efficacy of Inmazeb were evaluated in a multi-center, open-label, randomized controlled trial, in which 154 patients received Inmazeb (50 mg of each monoclonal antibody) intravenously as a single infusion, and 168 patients received an investigational control.
The primary efficacy endpoint was 28-day mortality. The primary analysis population was all patients who were randomized and concurrently eligible to receive either Inmazeb or the investigational control during the same time period of the trial.
Of the 154 patients who received Inmazeb, 33.8% died after 28 days, compared to 51% of the 153 patients who received a control. In the expanded access program, an additional 228 patients received Inmazeb.
The most common symptoms experienced while receiving Inmazeb included: fever, chills, tachycardia (fast heart rate), tachypnea (fast breathing), and vomiting; however, these are also common symptoms of Ebola virus infection. Patients who receive Inmazeb should avoid the concurrent administration of a live vaccine due to the treatment’s potential to inhibit replication of a live vaccine virus indicated for the prevention of Ebola virus infection and possibly reduce the vaccine’s efficacy.
Hypersensitivity, including infusion-related events, can occur in patients taking Inmazeb, and treatment should be discontinued in the event of a hypersensitivity reaction.
Previously, Inmazeb received an Orphan Drug designation for the treatment of Ebola virus infection. Additionally, the agency granted Inmazeb a Breakthrough Therapy designation for the treatment of Zaire ebolavirus infection.
From a prevention perspective, the FDA previously authorized Ervebo during December 2019.
The FDA is an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices.
PrecisionVaccinations publishes research-based Ebola treatment news.
Our Trust Standards: Medical Advisory Committee