These Ebola Vaccines Found Protective
While the world has recently focused on the Sudan ebolavirus outbreak in the Republic of Uganda and its lack of protective vaccines, a new study clarifies the value of other Ebolavirus strains vaccines.
Zaire Ebolavirus has been causing outbreaks in Africa with high mortality and morbidity for years.
Two vaccine strategies to prevent Ebola virus disease (EVD) have received World Health Organization (WHO) prequalification status and were used during the most recent outbreaks.
Since 2019, Zaire Ebola vaccines have been approved by the U.S. Food and Drug Administration (FDA), European Medicines Agency, and the U.K.
The rVSVΔG-ZEBOV-GP (Ervebo®) vaccine is designed as a one-dose vaccine and has been recommended for reactive ring vaccination use in persons at high risk for exposure during outbreaks.
The ring strategy is used to identify contacts and contacts of contacts.
And the Ad26.ZEBOV–MVA-BN-Filo (Zabdeno and Mvabea) combination is a two-dose regimen, with the vaccines administered 56 days apart, that the WHO has recently recommended for persons at some risk for EVD but are not considered to be at high risk.
A New England Journal of Medicine (NEJM) Original Article published on December 14, 2022, presented the Partnership for Research on Ebola Vaccinations (PREVAC) Study team's findings.
This NEJM article presented the results of two randomized, placebo-controlled trials with 1,400 adults and 1,401 children.
Among adults and children, the incidence of injection-site reactions and symptoms was higher the week after receipt of the primary and second or booster vaccinations than after receipt of placebo but not at later time points.
But these events were essentially low-grade.
At month 12 of the PREVAC study:
- a total of 41% of adults (titer, 401 EU per milliliter) and 78% of children (titer, 828 EU per milliliter) had a response in the Ad26–MVA group,
- 76% (titer, 992 EU per milliliter) and 87% (titer, 1415 EU per milliliter), respectively, had a response in the rVSV group,
- 81% (titer, 1037 EU per milliliter) and 93% (titer, 1745 EU per milliliter), respectively, had a response in the rVSV–booster group,
- 3% (titer, 93 EU per milliliter) and 4% (titer, 67 EU per milliliter), respectively, had a response in the placebo group (P<0.001 for all comparisons of vaccine with placebo).
- In adults and children, antibody responses with the vaccine differed from those with placebo beginning on day 14.
In summary, this PREVAC study found that all three vaccine regimens produced immune responses from day 14 through month 12.
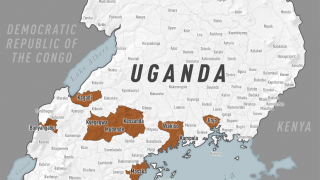
The WHO recently reported good news regarding Uganda's recent Sudan ebolavirus disease (SYDV) outbreak.
As of early December, there are no active SUDV cases.
Since the outbreak declaration on September 20 until December 5, 2022, the Uganda Ministry of Health reported a total of 142 confirmed cases.
Among them, 55 deaths occurred, leading to a CFR of 39%.
And the number of SUDV cases among healthcare workers remains unchanged, with 19 confirmed cases and seven deaths.
Vax-Before-Travel posts other SUDV outbreak news.
Additional Zaire vaccine and Sudan vaccine candidate news are posted at PrecisionVaccinations.com/Ebola.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee