The WHO Issues Strong Recommendations for Ebola Monoclonal Antibodies
The World Health Organization (WHO) published its first guideline for Ebola virus disease (EVD) therapeutics following a systematic review and meta-analysis of randomized clinical trials, with strong recommendations for using two monoclonal antibodies, Ebanga and Inmazeb.
The WHO stated on August 19, 2022, that EVD is a severe and too often fatal illness caused by the Ebola virus and called on the global community to increase access to these lifesaving monoclonal antibodies (mAbs) treatments.
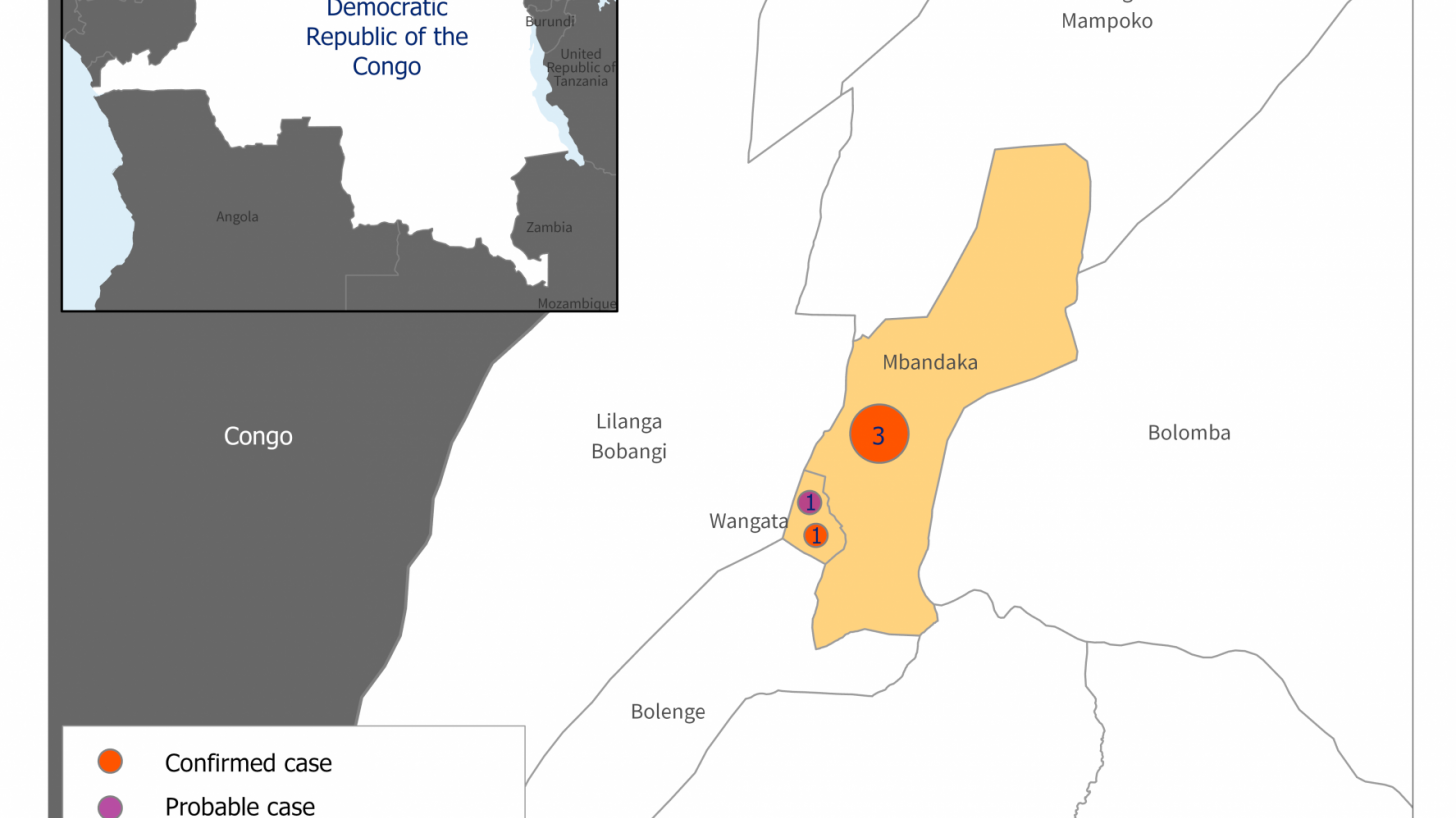
This is essential advice since the Democratic Republic of Congo (DRC) recently concluded its 14th EVD outbreak.
Between April and July 2022, five cases of EVD (case fatality ratio 100%) were reported from three health areas in the DRC's Equateur province.
Previous Ebola outbreaks and responses have shown early diagnosis and treatment with optimized supportive care.
"This therapeutic guide is a critical tool to fight Ebola," said Dr. Richard Kojan, co-chair of the Guideline Development Group of experts selected by WHO and President of ALIMA, The Alliance for International Medical Action, in a related media statement.
"From now on, people infected with the Ebola virus will have a greater chance of recovering if they seek care as early as possible."
"As with other infectious diseases, timeliness is key, and people should not hesitate to consult health workers as quickly as possible to ensure they receive the best care possible."
The two recommended therapeutics have demonstrated clear benefits and, therefore, can be used for all patients confirmed positive for EVD, including older people, pregnant and breastfeeding women, children, and newborns born to mothers with confirmed Ebola within the first seven days after birth.
Patients should receive recommended neutralizing mAbs as soon as possible after laboratory confirmation of diagnosis.
However, access to both these treatments remains challenging, especially in resource-poor areas.
These drugs should be where patients need them the most: where there is an active Ebola outbreak or where the threat of outbreaks is high or very likely, such as in the DRC.
Additionally, the WHO published the first invitation to therapeutics manufacturers against Ebola virus disease to share their drugs for evaluation by the WHO Prequalification Unit, a crucial step to improve drug access for communities and countries affected by Ebola.
Dr. Janet Diaz, lead of the clinical management unit in WHO's Health Emergencies program, commented, "Doing the basics well, including early diagnosis, providing optimized supportive care with the evaluation of new therapeutics under clinical trials, has transformed what is possible during Ebola outbreaks."
"This has led to developing a new standard of care for patients."
Although WHO was able to make strong recommendations for the use of two therapeutics, there is a need for further research and evaluation of clinical interventions, as many uncertainties remain.
There is also a recommendation on therapeutics that should not be used to treat patients, including ZMapp and remdesivir.
All these WHO recommendations only apply to EVD caused by the Zaire ebolavirus.
In addition to mAbs treatments, there are authorized vaccines that prevent Ebola infections, such as Merck's Ervebo.
In the USA, these Ebola treatments and vaccines are distributed by the government.
The U.S. CDC suggests speaking with a certified travel vaccination provider before visiting high-risk Ebola areas, such as the DRC.
During the DRC's recent outbreak, about 600,000 travelers registered at 16 points of entry.
Among those who were screened for EVD, 134 suspected cases were subsequently tested, and none tested positive for EVD.
Vax-Before-Travel publishes fact-checked, research-based travel vaccine news curated for mobile readership.
Our Trust Standards: Medical Advisory Committee


