Second Chikungunya Virus Vaccine Coming Soon
Bavarian Nordic A/S today announced the completion of the rolling submission process with the U.S. Food and Drug Administration (FDA) for a Biologics License Application (BLA) for the licensure of its CHIKV VLP vaccine candidate for immunization against chikungunya virus infection in individuals 12 years of age and older.
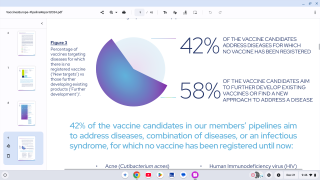
CHIKV VLP is an adjuvanted VLP-based vaccine candidate for active immunization against chikungunya disease.
Initiated in April 2024, with acceptance from the FDA, the BLA could support a potential vaccine approval in the first half of 2025.
Bavarian Nordic also intends to submit a Marketing Authorisation Application (MAA) with the European Medicines Agency (EMA) by the end of the first half of 2024. The MAA has already been granted accelerated assessment, which means the CHIKV VLP vaccine could obtain approval from the European Commission in the first half of 2025.
“The completion of the BLA submission marks a significant milestone in the development of our CHIKV VLP vaccine and represents an important contribution to the development of preventative solutions for individuals 12 years of age and older at risk of chikungunya virus from bites by infected mosquitos. With the near-term anticipated MAA submission to EMA, we are looking towards potential approval of the vaccine in the first half of 2025 and subsequent launch in both the U.S. and E.U.,” said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on June 17, 2024.
In the United States, the FDA has approved one chikungunya vaccine.
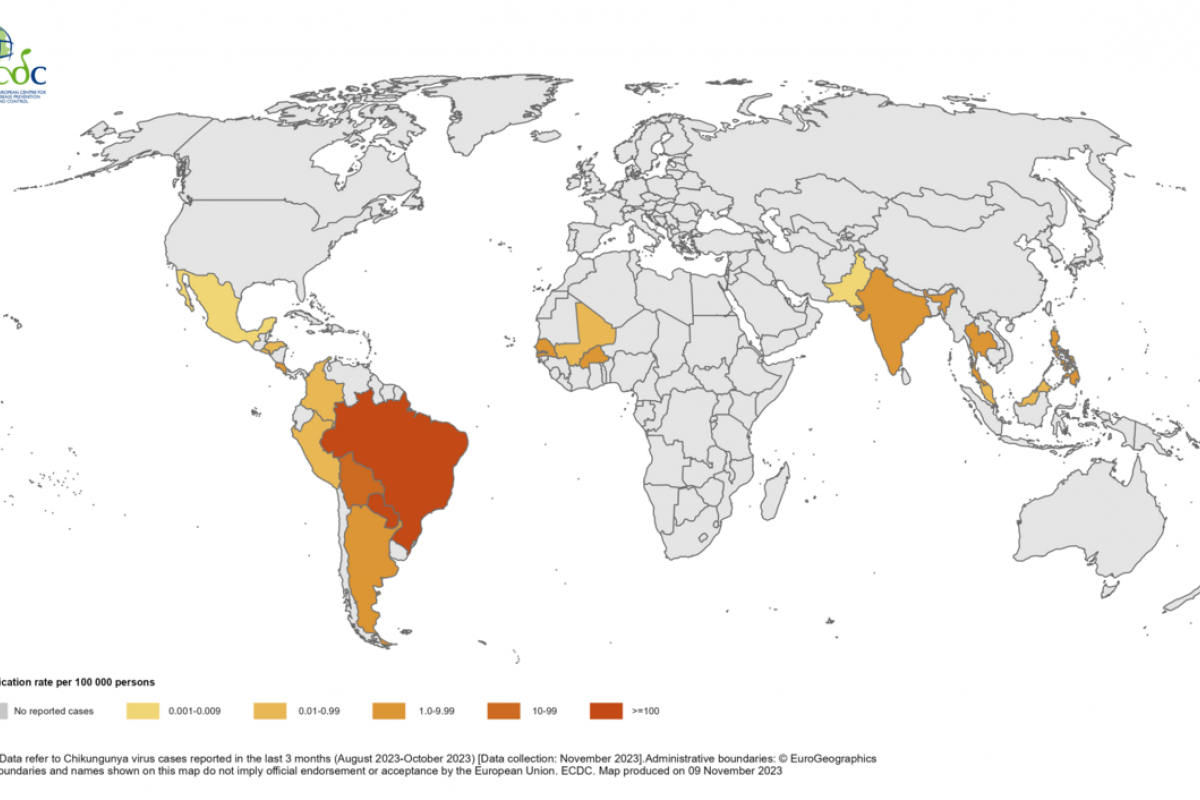
Chikungunya is a mosquito-borne viral disease that causes fever and severe joint pain. The World Health Organization (WHO) says the disease was first recognized in 1952. It is a ribonucleic acid virus belonging to the alphavirus genus of the family Togaviridae.
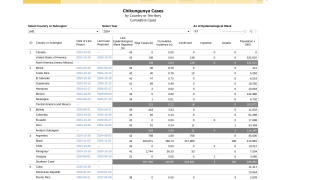
The WHO says chikungunya outbreaks have been identified in nearly 115 countries, primarily in the Region of the Americas. Most chikungunya cases in the contentital USA are travel related.
Our Trust Standards: Medical Advisory Committee