Blood-stage Malaria Vaccine Booster Complements Approved Vaccines

As two approved malaria vaccines ramp up production to meet demand in 2025, an innovative booster vaccine candidate has been found to enhance immunity and reduce the number of cases and related fatalities.
According to the results of a phase 2b clinical trial, the RH5.1/Matrix-MTM malaria vaccine was 55% effective in preventing clinical malaria over six months.
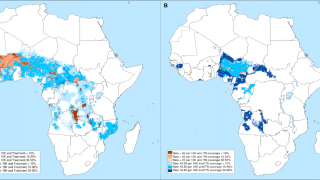
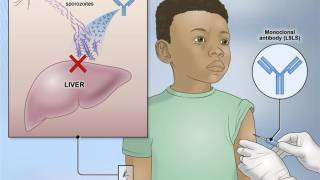
Unlike the previously approved malaria vaccines (RTS,S/AS01, and R21/Matrix-M) that target the pre-erythrocyte stage, RH5.1/Matrix-M targets blood-stage malaria.
In a Commentary published by The Lancet Infectious Diseases on January 7, 2025, the authors indicated that study participants vaccinated with RH5.1/Matrix-M showed high concentrations of anti-RH5.1 serum IgG antibodies and high levels of in-vitro growth inhibition activity against Plasmodium falciparum.
The vaccine candidate was also well tolerated, with only mild adverse events like fever and injection site swelling.
Moreover, the delayed third-dose regimen induced higher immune responses than the monthly regimen.
"We found this clinical trial finding encouraging for the fight against malaria, particularly in Africa. This blood-stage candidate vaccine RH5.1/Matrix-M will complement the previously WHO-recommended and rolled out pre-erythrocyte malaria vaccines for effective and durable protection, targeting different stages of the parasite life cycle," wrote these authors.
On December 11, 2024, Professor Angela Minassian, an infectious diseases physician and Associate Professor in the Department of Biochemistry who leads the clinical blood-stage malaria vaccine program at the University of Oxford, said in a press release, 'Our goal, by targeting the blood stage of the disease with this vaccine, is to reduce the number of severe cases and deaths significantly.'
"The currently licensed vaccines target the liver stage of the parasite and are very effective at stopping parasites from getting into the blood."
"However, if they fail and parasites slip through the net, the disease will develop as these approved vaccines have no activity against malaria in the blood."
"Adding RH5.1/Matrix-MTM to these licensed vaccines should provide a vital second line of defense, achieving even higher levels of protection. Importantly, our study has provided the first real-world data to show that this type of vaccine works by reducing the level of parasites in the blood.'
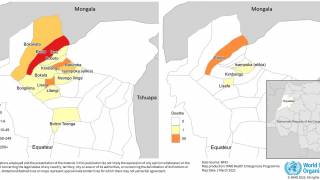
This clinical study, which is ongoing in Burkina Faso, has been run by scientists at the University of Oxford in collaboration with the Serum Institute of India Pvt. Ltd, Novavax, and others.
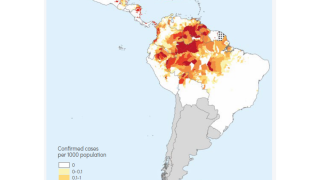
The majority of malaria cases in the United States are travel-related.
As of the week ending November 23, the U.S. CDC confirmed 1,772 malaria cases during 2024, mostly in international travelers arriving in New York City, Miami, Florida, and Los Angeles, California.
While the approved malaria vaccines are offered in various African countries, they are not U.S. FDA-approved and are unavailable at travel clinics and pharmacies in January 2025.
Our Trust Standards: Medical Advisory Committee