Most Countries Confirmed Fewer Zika Infections in 2024

Since first impacting people in the Region of America in 2014, the mosquito-transmitted Zika virus has affected many people's health. According to recent reports, most countries in the Americas reported fewer Zika outbreaks in 2024.
Additionally, a second-generation Zika vaccine candidate has progressed in clinical trial development for the first time.
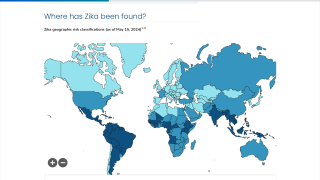
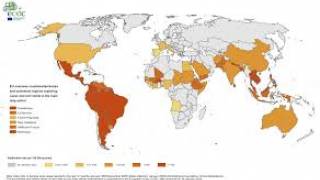
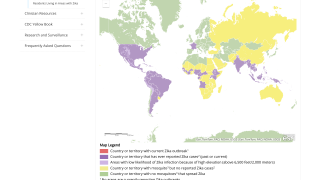
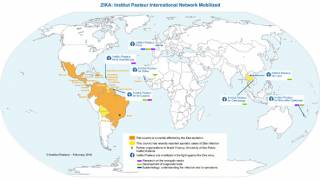
As of January 3, 2024, the Pan American Health Organization (PAHO) confirmed the local transmission of Zika in countries and territories in the Americas region.
The PAHO reported over 41,942 ZIka cases and two related fatalities in the Americas in 2024, with the highest proportion of Zika cases reported in Brazil, Bolivia, and Columbia.
In December 2024, Brazil was the leading country reporting increased Zika cases. Brazil's Ministry of Health indicated over 40,000 cases in 2024, compared to 35,962 in 2023.
In the United States, the U.S. CDC only reported 19 non-congenital Zika cases in U.S. residents (1 imported case in Texas).
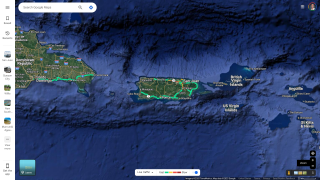
And in the U.S. Territories, Puerto Rico has been categorized as having a risk of Zika virus transmission for several years. As of December 2024, 16 Zika cases have been reported, a decrease from 2023, when 43 cases were reported.
Unfortunately, the Zika virus has been reported in several of India's 16 states/union territories, such as Maharashtra, for several years, and 2024 continued this trend.
To alert international travelers, the CDC issued a Level 2—Practice Enhanced Precautions regarding India's ongoing Zika outbreak in August 2024.
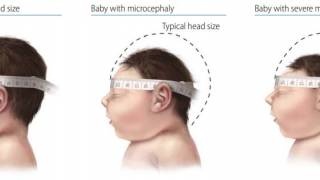
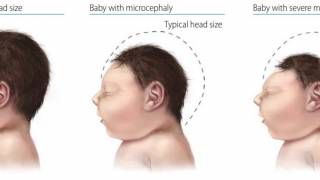
The CDC says most people infected with Zika will not have symptoms or mild symptoms, such as fever, rash, headache, joint and muscle pain, and red eyes. However, infection during pregnancy can cause certain birth defects, such as microcephaly.
From a disease prevention perspective, Valneva SE's VLA1601, a purified, inactivated, whole Zika vaccine candidate adsorbed on aluminum hydroxide, is the most advanced vaccine development in 2025.
Topline data from Valneva's phase 1 clinical trial (VLA1601-102) are expected in 2025. The company previously stated that it plans to accelerate vaccine approval discussions with government agencies like the U.S. FDA.
Our Trust Standards: Medical Advisory Committee