Approved Chikungunya Vaccine Confirmed Safe and Effective

As the winter season approaches in the United States, many people plan to vacation in the warmer weather of the Southern Hemisphere this year.
Since mosquitoes actively transmit various diseases in the Caribbean, Central, and South America, protecting yourself with a safe and effective vaccine one month before traveling south is an intelligent goal.
The Journal of Travel Medicine published a pooled analysis from clinical trials on October 14, 2024, that confirmed the recently approved Chikungunya virus vaccine 'presented with an excellent local tolerability profile and overall safety in line with that expected for a live-attenuated vaccine.'
This positive report complements various studies that have concluded Valneva SE's IXCHIQ® monovalent, single-dose, live-attenuated chikungunya vaccine is very effective.
Furthermore, most vaccine efficacy studies published over the past year have reported that IXCHIQ is over 90% protective just two weeks after admission.
The U.S. CDC recommends vaccination for adults traveling to a country with a Chikungunya outbreak, such as Brazil, Bolivia, and Paraguay.
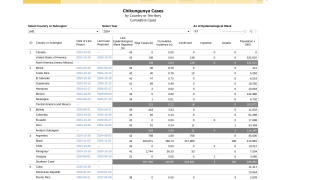
With about 400,000 cases and 177 related fatalities already reported in the Region of the Americas this year, getting vaccinated is an easy decision.
As of October 15, 2024, IXCHIQ was available for adults in the U.S., Canada, Europe, and the Virgin Islands, often offered at travel clinics and pharmacies.
Our Trust Standards: Medical Advisory Committee