Visitors At Risk During Arizona's Valley Fever Outbreak
A seldom discussed fungal infection, primarily found in the soil in the Southwest and endemic to Arizona, significantly impacted people's respiratory health in 2024. Living in Maricopa County, which includes Phoenix, puts residents at high risk for Valley fever.
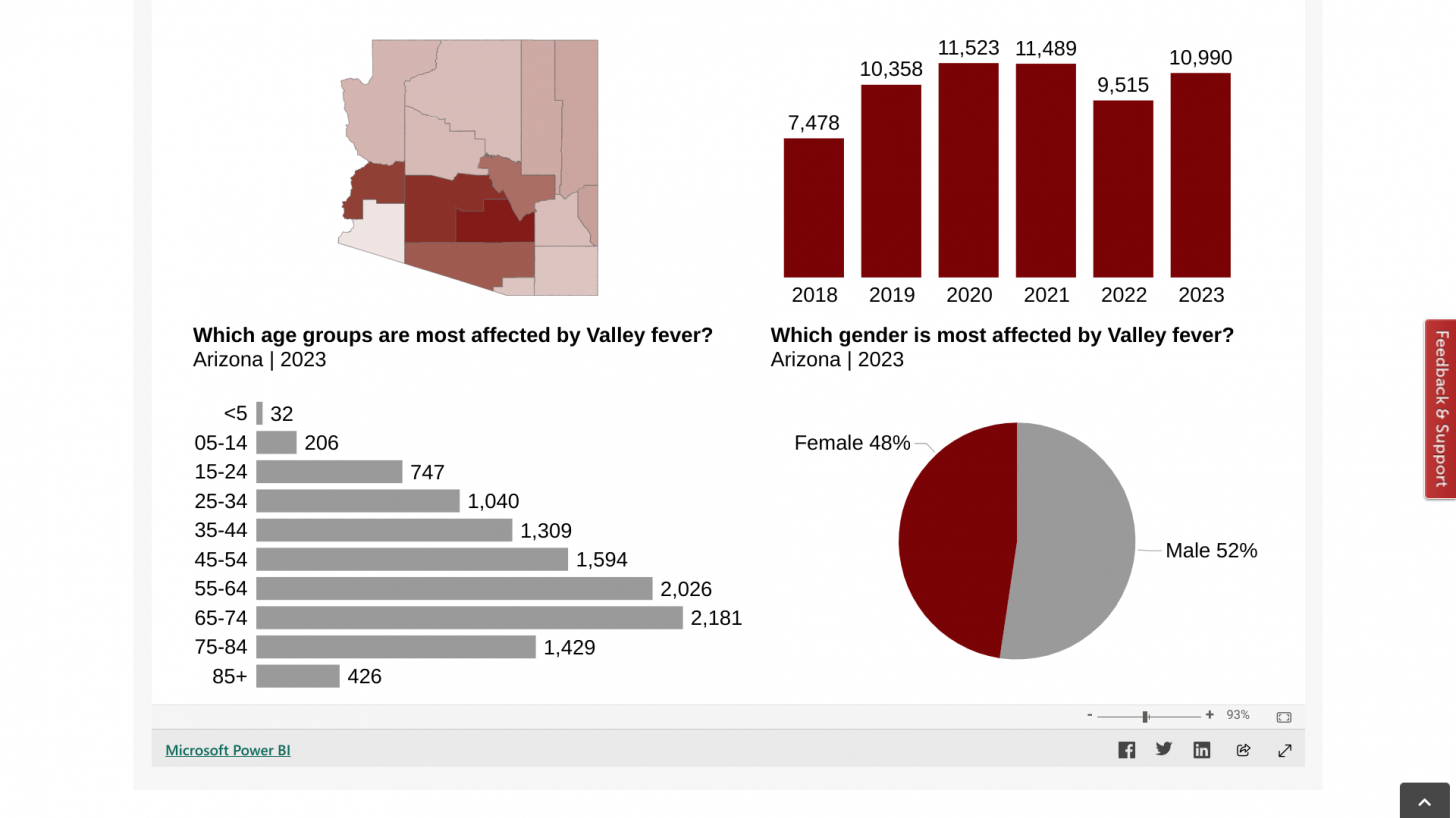
While anyone who lives in or travels to a Valley Fever (coccidioidomycosis) endemic area is at risk, with about 5% of seniors most vulnerable.
According to data posted by the Arizona Department of Health Services (ADHS) on January 3, 2025, there have been about 100% more Vallery fever cases per year over the past decade.
As of the end of 2024, ADHS reported that Maricopa County had about 9,744 of Arizona's 14,680 Valley fever cases. In 2023, all of Arizona reported 9,148 cases.
Throughout the United States, 22,939 cases were reported in 2024. On average, there were approximately 200 coccidioidomycosis-associated fatalities each year in the U.S.
Without an approved antifungal vaccine for humans in 2025, Valley Fever infections require immediate treatment and can become very serious. Symptoms can last for years and may require lifelong treatment with antifungal medication.
Infections can cause chronic pneumonia, similar to tuberculosis, and disseminated disease.
Disseminated disease is when the fungal infection spreads from the lungs to other body parts. The most common areas in which it spreads are the skin, bones, joints, and brain. Infection of the brain can be deadly.
While the U.S. CDC has not issued a Travel Health Advisory regarding Valley fever outbreaks in Arizona and California, countries such as Canada list this disease as a health risk when visiting the U.S.
From a disease prevention perspective, Anivive Lifesciences Inc. recently announced that the National Institute of Allergy and Infectious Diseases has awarded a new contract worth up to $33 million to support the development of a vaccine against Coccidioides.
Dr. Edward Robb, Anivive Lifesciences Chief Strategy Officer and Principal Investigator commented in a press release on August 2, 2024, "This collaborative effort has delivered a significant step forward in the field of vaccinology and holds the potential to be the first vaccine to prevent a serious systemic fungal infection common to humans and animals."
This contract funding aims to utilize the underlying science in Anivive's animal health Valley Fever vaccine for dogs, currently under review by the USDA Center for Veterinary Biologics.
Our Trust Standards: Medical Advisory Committee
