Chikungunya Outbreaks Approaching Unfortunate Record in 2024
The rise in chikungunya fever outbreaks and their spread to more than 100 geographical areas pose a significant public health challenge worldwide. In 2024, this mosquito-transmitted viral illness continues impacting people in the Region of the Americas.
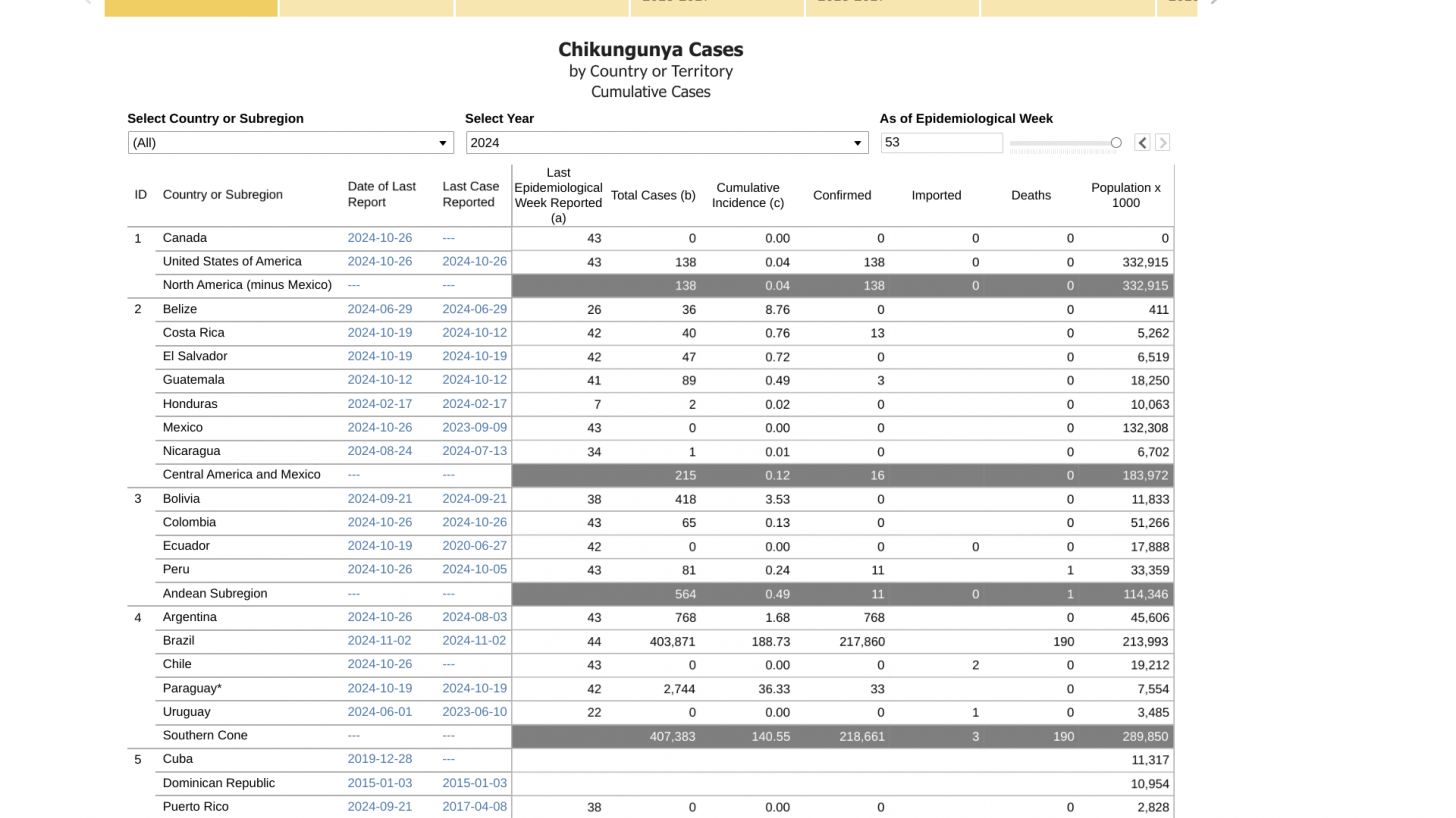
New data from the Pan American Health Organization (PAHO) indicates that the number of chikungunya virus (CHIKV) cases reported in 2024 may reach an unfortunate record high.
As of November 7, 2024, the PAHO's data dashboard indicates there have been 408,310 CHIKV cases and 191 related deaths in the Americas this year. This compares with 411,548 instances for all of 2023 and just 273,841 in 2022.
Even in the northern section of the Americas, chikungunya cases are accelerating.
As of November 2024 (Week 43), the U.S. Centers for Disease Control and Prevention (CDC) reported 138 travel-related chikungunya cases led by Massachusetts (20), New Hampshire, California, Colorado, Illinois, Texas, and New York.
From 2006 to 2023, 4,590 travel-related cases were reported in the U.S., with 118 reported in 2023. Most of these infected international travelers had visited countries in Central and South America.
This virus is transmitted to humans by mosquitoes infected with CHIKV. The mosquitoes involved are Aedes aegypti and Aedes albopictus.
Recent research indicates these mosquitoes are steadily expanding northward and being found at higher elevations.
The most common symptom of an infection is an abrupt onset of fever, often accompanied by joint pain. While serious complications are uncommon, atypical severe cases can cause long-term symptoms, especially in older people and children.
Recent research found that seven years after infection, 14.1% of study participants were experiencing CHIKV symptoms, and 41% had non-inflammatory pain that impacted their quality of life.
These researchers wrote in October 2024, 'Our (study) results showed that about one out of seven patients with CHIKV infection developed symptoms and signs compatible with chronic rheumatism almost eight years post-disease onset.'
To protect international travelers from CHIKV infection, the U.S. FDA approved Valneva SE's IXCHIQ® vaccine in late 2023. This is the only chikungunya vaccine commercially available at travel clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee