Chikungunya Symptoms Detected Seven Years Later

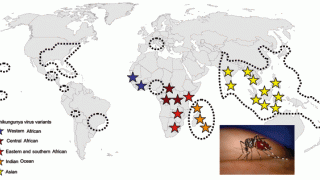
Throughout 2024, the Chikungunya alphavirus has caused outbreaks in many parts of the world, including Africa, the Americas, Asia, Europe, and islands in the Indian and Pacific Oceans.
For example, the Pan American Health Organization reported on November 4, 2024, that there have been over 404,000 Chikungunya cases and 185 related fatalities in the Americas this year.
While the U.S. Centers for Disease Control and Prevention (CDC) says Chikungunya virus (CHIKV) infections can cause a short-term illness with fever and joint pain, many people have reported lingering symptoms.
This study, published by MDPI on October 19, 2024, determined the incidence of post-chikungunya chronic rheumatism (pCHIK-CR) and its impact on quality of life (QoL) and chronic fatigue in adults seven years after the 2014–2015 CHIKV outbreak in Colombia. This study implemented a comprehensive clinical assessment to objectively estimate and characterize the incidence of chronic rheumatological disease attributed to CHIKV infection.
Seven years after infection, 14.1% of participants were classified as pCHIK-CR cases, 41% as having non-inflammatory pain, likely degenerative (NIP-LD), and 44.9% without rheumatic disease (Wo-RM).
Patients with pCHIK-CR and NIP-LD exhibited significantly worse QoL compared to Wo-RM cases.
Chronic fatigue prevalence increased from 8.6% in Wo-RM patients to 25% in NIP-LD and 54.6% in pCHIK-CR cases. One in seven cases with CHIKV infection develops pCHIK-CR, which impacts both QoL and chronic fatigue.
These researchers wrote, 'Our results showed that about one out of seven patients with CHIKV infection developed symptoms and signs compatible with chronic rheumatism (pCHIK-CR) almost eight years post-disease onset.'
This condition leads to a significant impairment of QoL and a high prevalence of chronic fatigue, comparable to that observed in non-inflammatory rheumatic diseases.'
To prevent CHIKV infections, the U.S. FDA approved a vaccine in 2023. Valneva SE's monovalent, single-dose, live-attenuated vaccine IXCHIQ® vaccine is commercially available at travel clinics and pharmacies in the U.S.
Our Trust Standards: Medical Advisory Committee