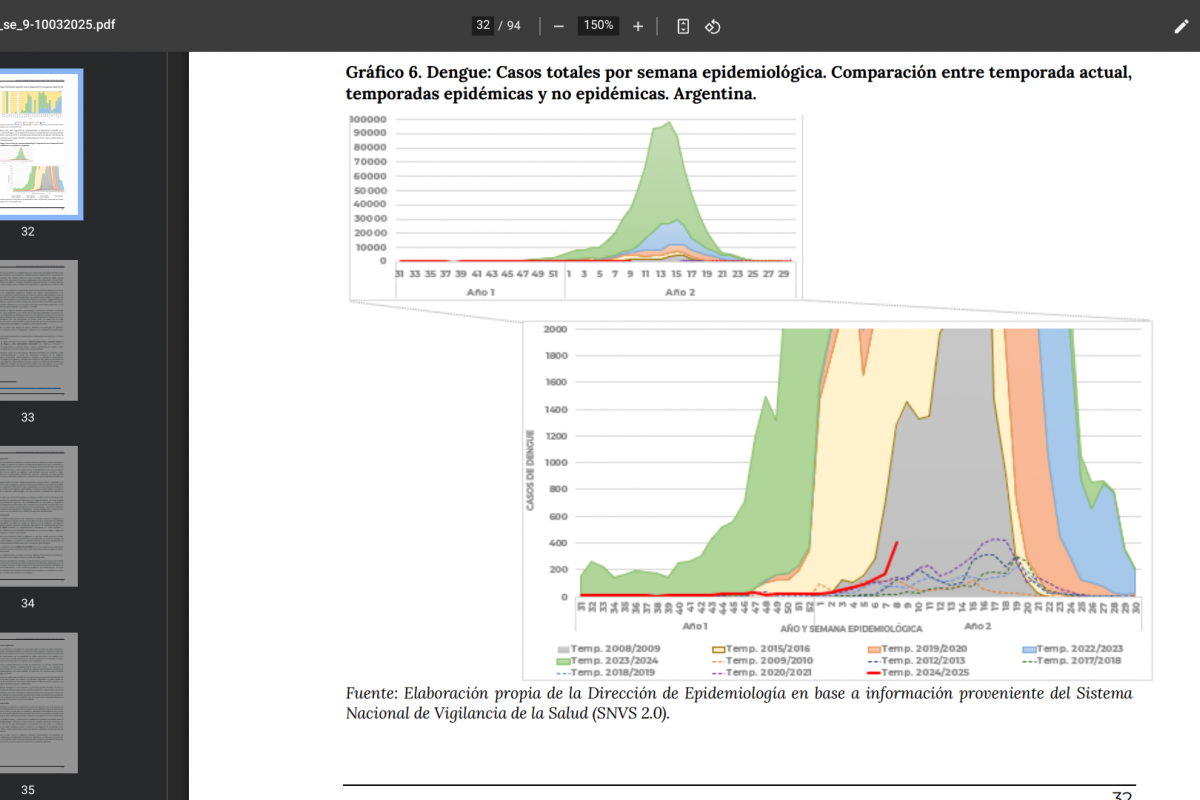
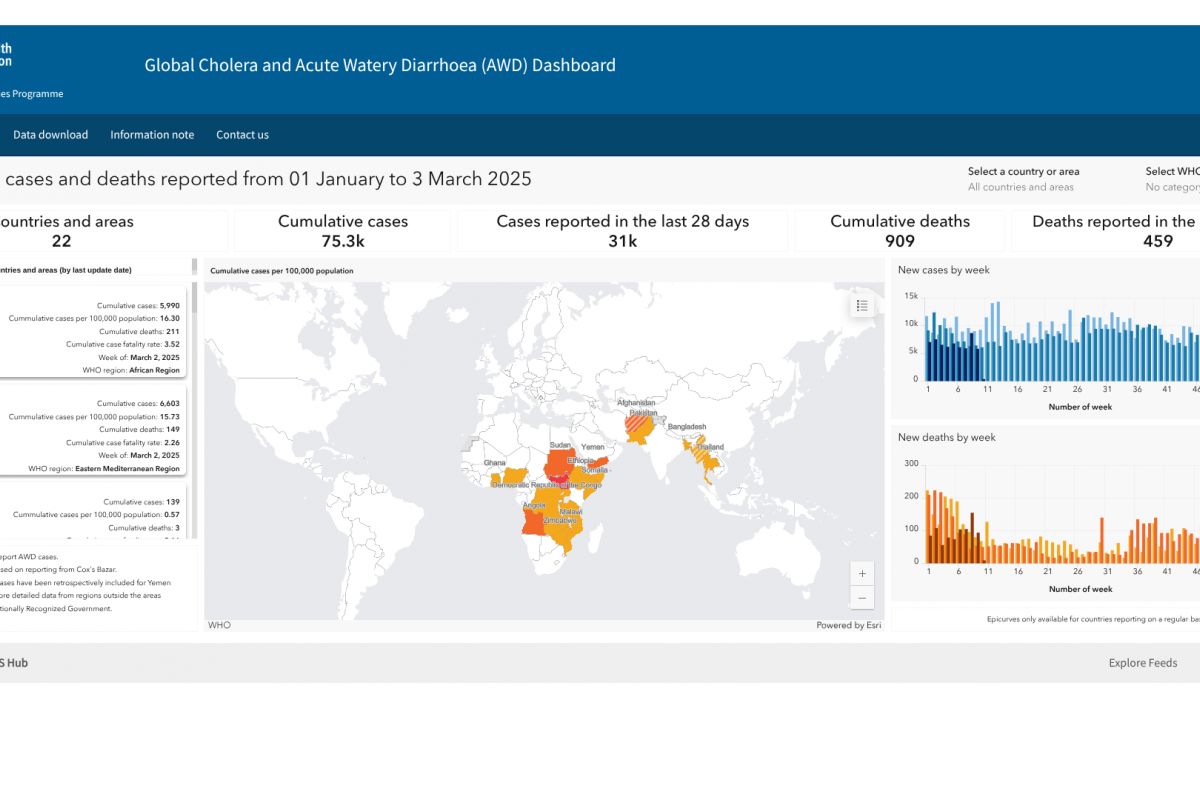
After a measurable decline in malaria cases globally over the last two decades, case numbers have rebounded throughout Africa during the previous two years, highlighting the need for enhanced prevention and treatment options.
According to the World Health Organization (WHO), despite the expenditure of $4 billion per year, malaria fatalities have not substantively been reduced during outbreaks.
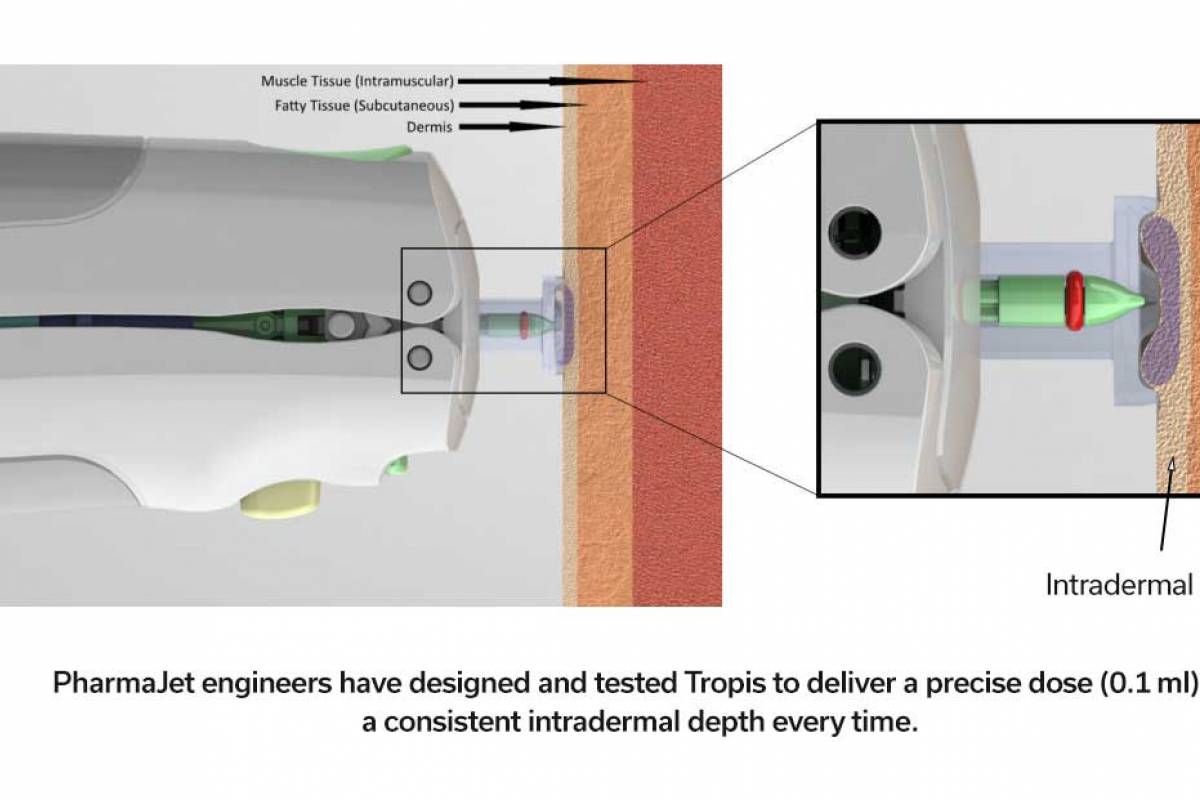
While the WHO recommends two malaria vaccines (Mosquirix™ and R21 / Matrix-M™) to reduce mosquito-transmitted malaria outbreaks in Africa, a new study has identified a potential change in case management.
In The Lancet Infectious Diseases, Virak Eng and colleagues provide evidence of the benefit of high total-dose primaquine (7 mg/kg) compared with low total-dose primaquine (3·5 mg/kg) to prevent relapsing P vivax malaria in Cambodia.
These findings, published on March 17, 2025, and funded by the U.S. National Institutes of Health, provide strong evidence for the optimal primaquine dose for anti-relapse therapy and support the 2024 WHO malaria treatment guidelines update recommending high-dose primaquine in most endemic countries.
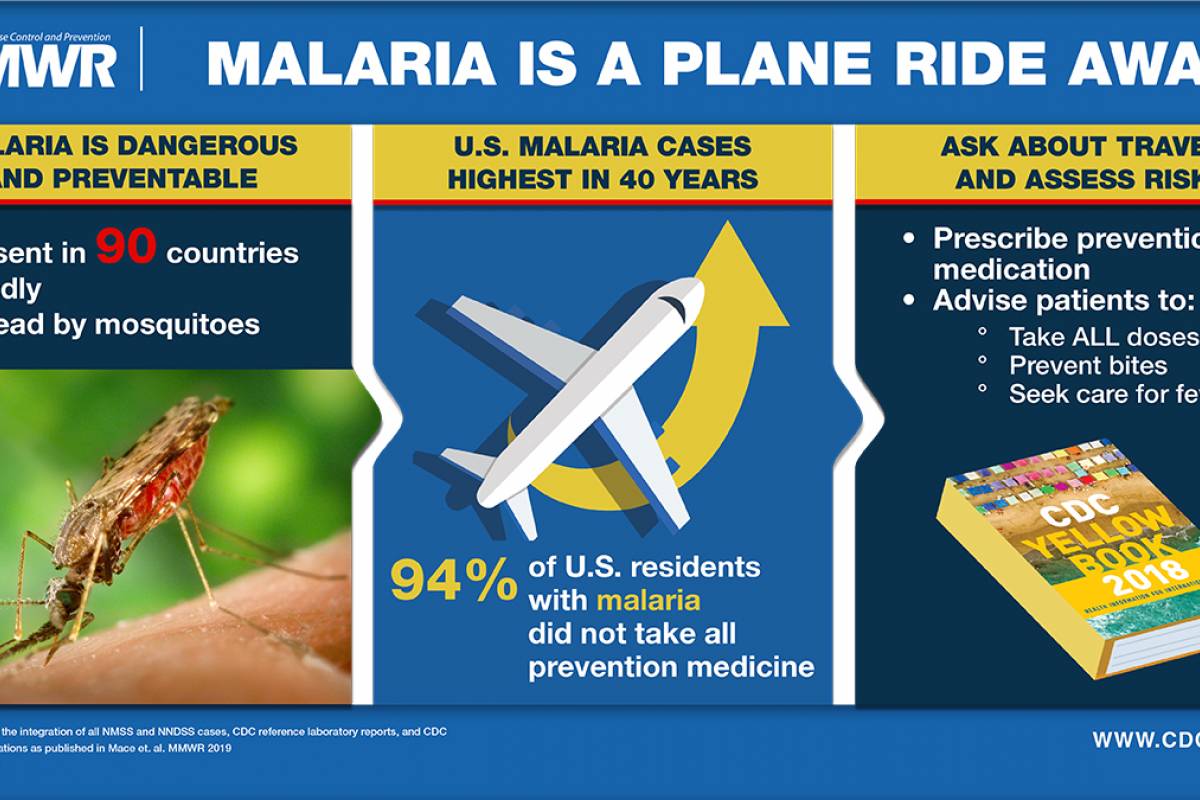
In the United States, most malaria cases are international travel-related, not locally transmitted.
Previously, the WHO estimated the annual global demand for malaria vaccines at 40–60 million doses by 2026. These vaccines are not commercially available in the U.S.