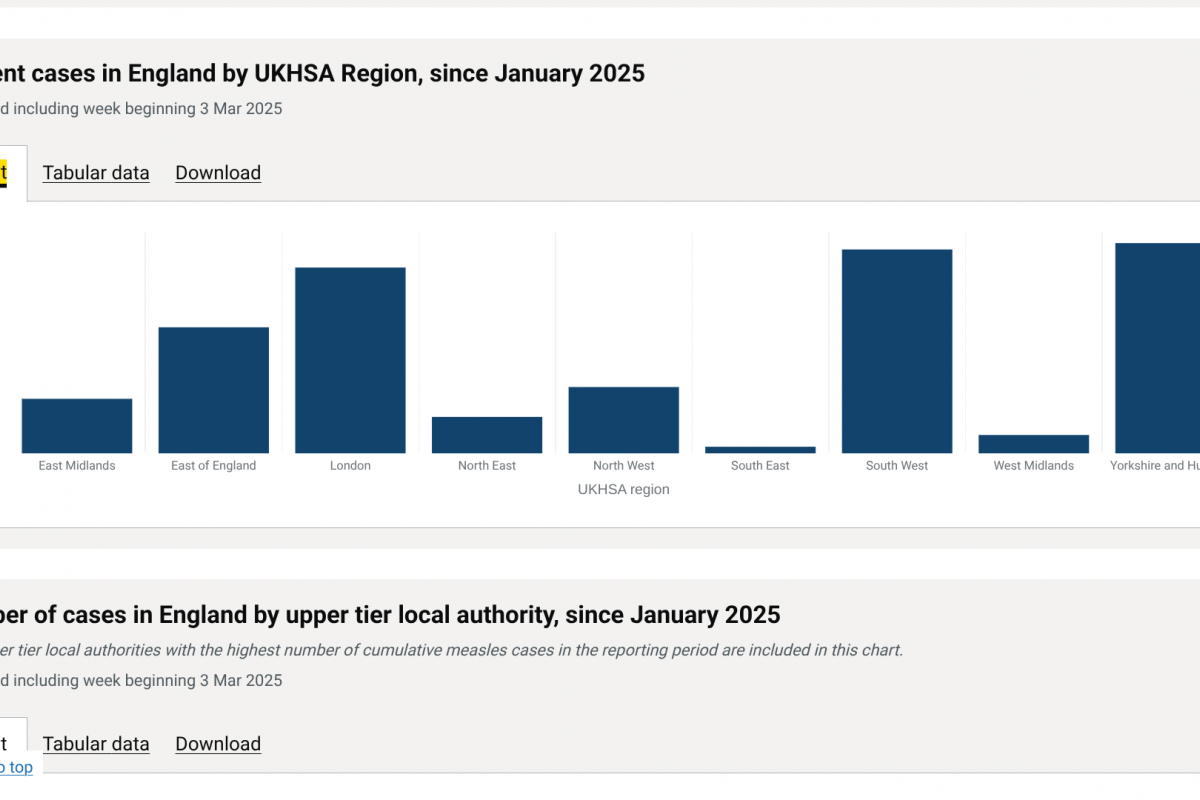
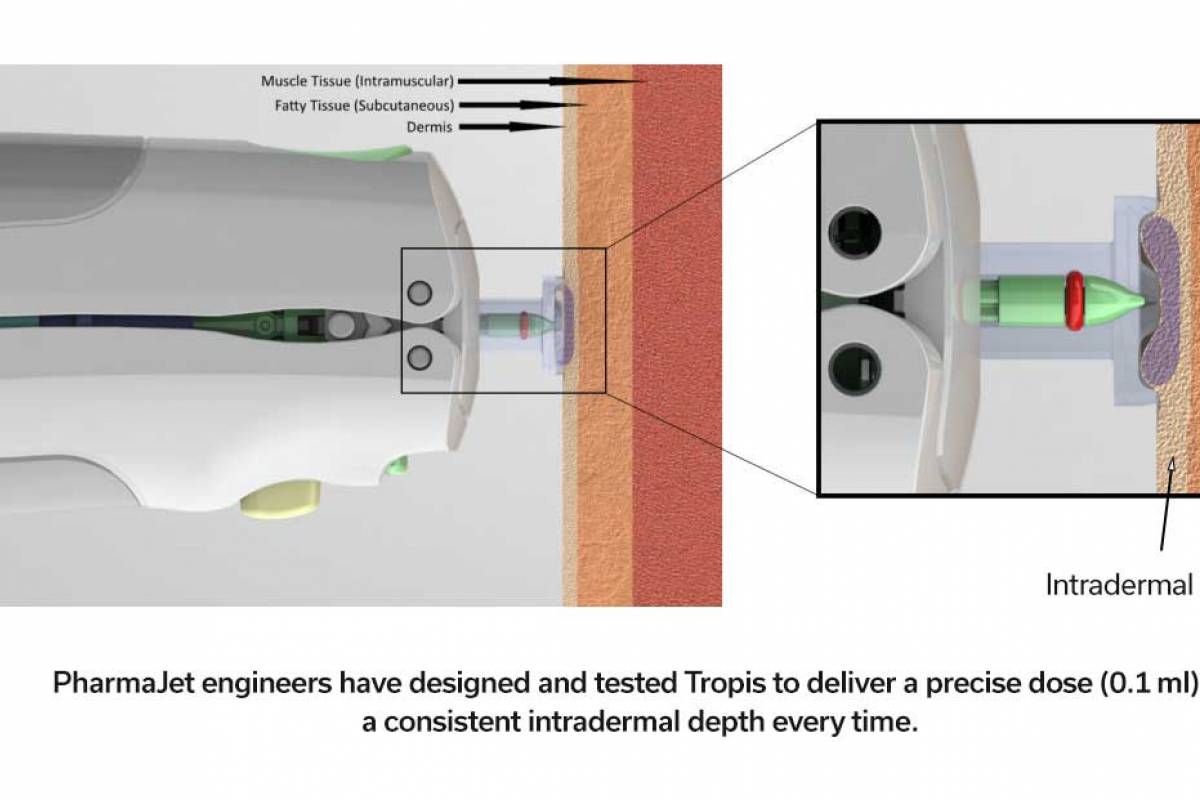
Since the U.K. Health Security Agency (UKHSA) declared a national measles incident in parts of England in 2024, cases have been reported in various cities besides London.
So far in 2025, 151 measles cases have been confirmed in Yorkshire and Humber, in the South West, Bristol, Leeds, Hertfordshire, and in London.
As of March 13, 2025, no acute measles-related deaths have been reported in 2025.
Last year, the UKHSA reported the most measles cases (2,911) in England in over a decade.
To England's east, 127,350 people were diagnosed with measles in Europe in 2024, led by Romania (27,568). The WHO says this is the highest number of measles cases in over 25 years.
“Measles is back, and it’s a wake-up call.... without high vaccination rates, there is no health security,” warned Dr Hans Henri P. Kluge, WHO Regional Director for Europe, in a media statement.
“The measles virus never rests – and neither can we.”
While the U.S. Centers for Disease Control and Prevention (CDC) recent Travel Health Notice identifies 57 countries reporting measles cases, it does not mention England.
However, the CDC does suggest that anyone visiting a measles outbreak area be fully protected from this contagious virus with the MMR vaccine, which is generally available at clinics and pharmacies in the U.S.