Cholera Returns to the U.S.

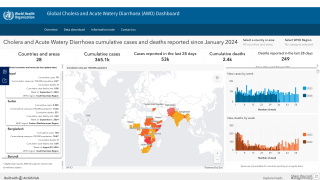
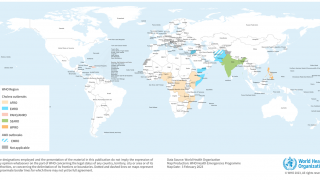
The U.S. Centers for Disease Control and Prevention (CDC) announced via email today that it has identified an unprecedented global increase in cholera infections, with outbreaks occurring in Haiti, Malawi, and Syria.
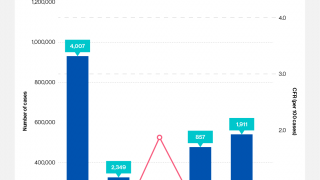
Thus far in 2022, the CDC reported (8) travelers infected with cholera have returned to the U.S. from Pakistan, Iraq, and Bangladesh.
Most persons infected with the cholera bacterium have mild diarrhea or no symptoms.
Only a small proportion, about 10%, of persons infected with Vibrio cholerae O1 may have an illness requiring treatment at a health center.
However, according to the CDC, cholera-related fatalities can occur within hours without treatment.
People who develop watery diarrhea within five days of being in any country where cholera outbreaks occur should seek medical care immediately.
Although cholera in travelers is rare and sustained community transmission in the U.S. is unlikely, widespread cholera outbreaks in other countries highlight the need for clinicians to be prepared to treat travelers with cholera.
Currently, the CDC considers 25 countries in Asia, Africa, and Haiti to have active cholera transmission.
Clinicians evaluating patients with acute onset of watery diarrhea should consider cholera in patients returning from affected regions, obtain a stool specimen for testing, and begin prompt treatment.
Cholera Information for Public Health and Medical Professionals, including how to treat cholera patients, is posted at this CDC link.
Furthermore, the CDC says pharmacies and medical facilities should have an ample supply of rehydration products.
In conjunction with hydration, treatment with antibiotics is recommended for severely ill patients.
When available, supplementation (20 mg zinc per day) in children six months or older should be considered, says the CDC.
Additionally, the CDC recommends vaccination for people traveling to or living in areas of active cholera transmission.
Unfortunately, the maker of the U.S. FDA-approved Vaxchora® (CVD 103-HgR) cholera vaccine temporarily stopped making and selling it in December 2020. Therefore, this vaccine is currently unavailable.
The World Health Organization has prequalified three other oral cholera vaccines.
But, these vaccines (Dukoral, ShanChol, Euvichol-Plus/Euvichol) are not available in the U.S.
To learn more about cholera vaccines, please visit Vax-Before-Travel.
PrecisionVaccinations publishes fact-checked, research-based vaccine information manually curated for mobile readers.
Our Trust Standards: Medical Advisory Committee