Cholera Vaccine Shortage Puts Millions of Children At-Risk

The International Coordinating Group (ICG) on Vaccine Provision has urged the authorities to address the unprecedented increase in cholera cases worldwide.
Today's recommended actions include investing in safe water, sanitation, and hygiene, improving the quality of and access to healthcare, and detecting and responding to outbreaks quickly.
The World Health Organization (WHO) confirmed on March 20, 2024, that the current trends are tragic because cholera is a preventable and treatable disease.
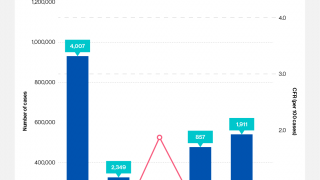
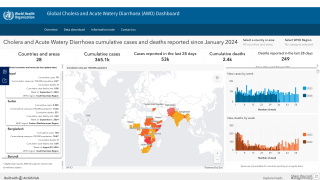
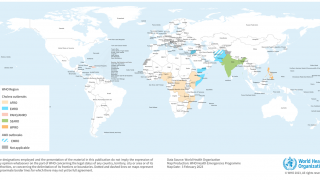
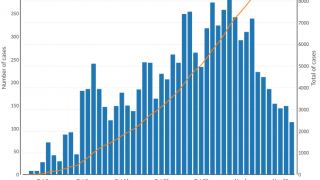
The persistence of cholera is evident as 2024 began, with 40,900 cases and 775 deaths reported (Edition #11) in January from 17 countries across four WHO regions.
Children under the age of five bear the brunt of this disease.
Preliminary data for 2023 reveal that over 700,000 cases were reported, and several of the outbreaks had high case fatality rates, exceeding the 1% threshold used as an indicator for early and adequate treatment of cholera patients.
The WHO has also recommended accelerating the production of oral cholera vaccines (OCVs) to prevent further cases.
Global production capacity in 2024 is forecast to be 37-50 million doses but will likely continue to be inadequate to serve the needs of millions of people directly affected by cholera.
The severe gap in the number of available vaccine doses, compared with the level of current need, puts unprecedented pressure on the global stockpile of vaccines. Between 2021 and 2023, more doses were requested for outbreak response than in the entire previous decade.
In October 2022, the ongoing vaccine shortage necessitated the ICG to recommend a single vaccine dose, down from a previous, long-standing two-dose regimen.
Approximately 36 million doses were produced in 2023, while 14 affected countries registered a need for 72 million doses for a one-dose reactive strategy.
These requests understate the actual need, says the WHO.
Preventive vaccination campaigns have had to be delayed to preserve doses for emergency outbreak control efforts, creating a vicious cycle.
The change in strategy enabled available vaccines to protect more people and respond to more cholera outbreaks amid the ongoing supply shortfall, but a return to a two-dose regimen and a resumption of preventive vaccination would provide more extended protection.
As of March 2024, the WHO has qualified Dukoral®, Shanchol™, and Euvichol® cholera vaccines.
New vaccine manufacturers are not expected to join the market before 2025; they must be fast-tracked, says the WHO.
Our Trust Standards: Medical Advisory Committee