Cholera Pandemic Continues With Constrained Vaccine Supply
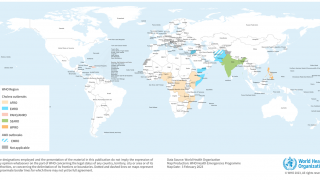
Since the 7th cholera pandemic began in Asia in 1961, about 45 countries have reported outbreaks this year. Unfortunately, this multi-decade pandemic is being contested without an ample supply of vaccines.
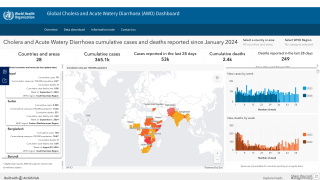
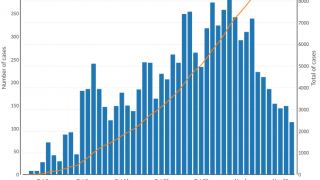
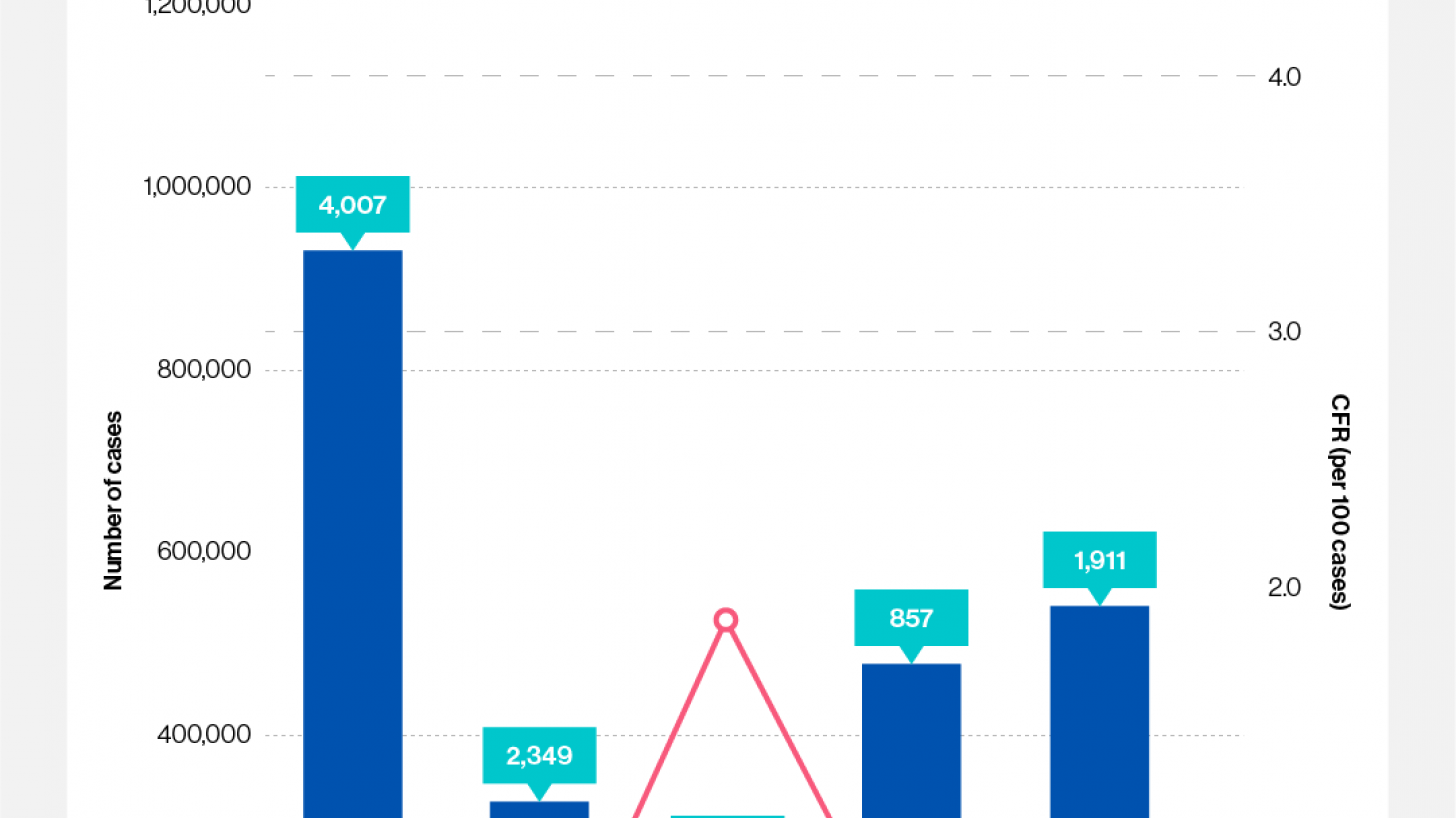
According to recent data from the World Health Organization (WHO), the number of cholera cases in 2024 may surpass the peak recorded five years ago. This projection is based on 2023 data showing a 13% increase in reported cholera cases compared to 2022.
On September 13, 2024, Linda Geddes from GAVI reported that not only had the number of countries affected by cholera outbreaks increased, but also the number experiencing large outbreaks. Nine countries reported 10,000 or more cases: Afghanistan, Bangladesh, the Democratic Republic of the Congo, Ethiopia, Haiti, Malawi, Mozambique, Somalia, and Zimbabwe.
Fortunately, most cholera cases reported in the United States are acquired during international travel. As of August 17, 2024, just six cholera cases were reported this year. In 2023, there were 10 cases.
Although oral cholera vaccine (OCV) supplies have been strained, a record 35 million doses were shipped to 12 countries in 2023.
To appropriately manage access to the limited OCV supply, the International Coordinating Group for OCVs was created in 1997. As of May 2024, the global vaccine production capacity is forecast to be 37-50 million doses, short of demand.
Since January 2023, 18 countries have requested 105 million OCV doses, nearly double the 55 million doses produced in that period.
The WHO says all OCVs require two doses to ensure protection for up to three years.
However, to streak the limited supply, GAVI says that during the current outbreak, only one OCV dose protocol has been implemented in reactive vaccination campaigns.
The WHO has recommended another OCV to address this global shortage. Euvichol-S is expected to be on the market at the end of 2024.
Currently, approved OCVs such as Dukoral® have increased production to alleviate the global inventory shortage.
Valneva SE’s global manufacturing network includes three in-house operations for the internal and external production of clinical and commercial products. In 2024, Valneva’s new manufacturing site in Sweden underwent regulatory evaluation and approval.
As of September 17, 2024, the Bavarian Nordic Vaxchora® single-dose vaccine is available in the U.S. and Canada.
Vaxchora (CVD 103-HgR) is indicated for active immunization against disease caused by Vibrio cholerae serogroup O1 in adults 18 through 64 years of age traveling to an active cholera-affected area.
In the U.S., Vaxchora is available in 2024 at specific travel clinics and pharmacies, such as Passport Health-Tampa.
Our Trust Standards: Medical Advisory Committee