17% of Canadians Vaccinated with Updated COVID-19
The Honourable Mark Holland, Canada's Minister of Health, issued a statement today marking the fourth National Day of Observance for COVID-19.
'Today's landscape is different than it was during the pandemic. COVID-19 vaccines and treatments are now more accessible to people in Canada, making it easier for everyone to protect themselves and their families from serious illness.'
Also, on March 11, 2024, Canada's National Advisory Committee on Immunization released updated guidance regarding the use of Nuvaxovid™ XBB.1.5, a subunit COVID-19 vaccine produced by Novavax Inc.
According to the committee's latest recommendations, announced on March 11, 2024, Novavax Inc.'s protein-based Nuvaxovid XBB.1.5 can be administered to individuals aged 12 and older, regardless of whether they have previously been vaccinated.
This recommendation supports efforts to provide greater access to a non-mRNA protein-based COVID-19 vaccine option and could help achieve improved immunization rates in Canada.
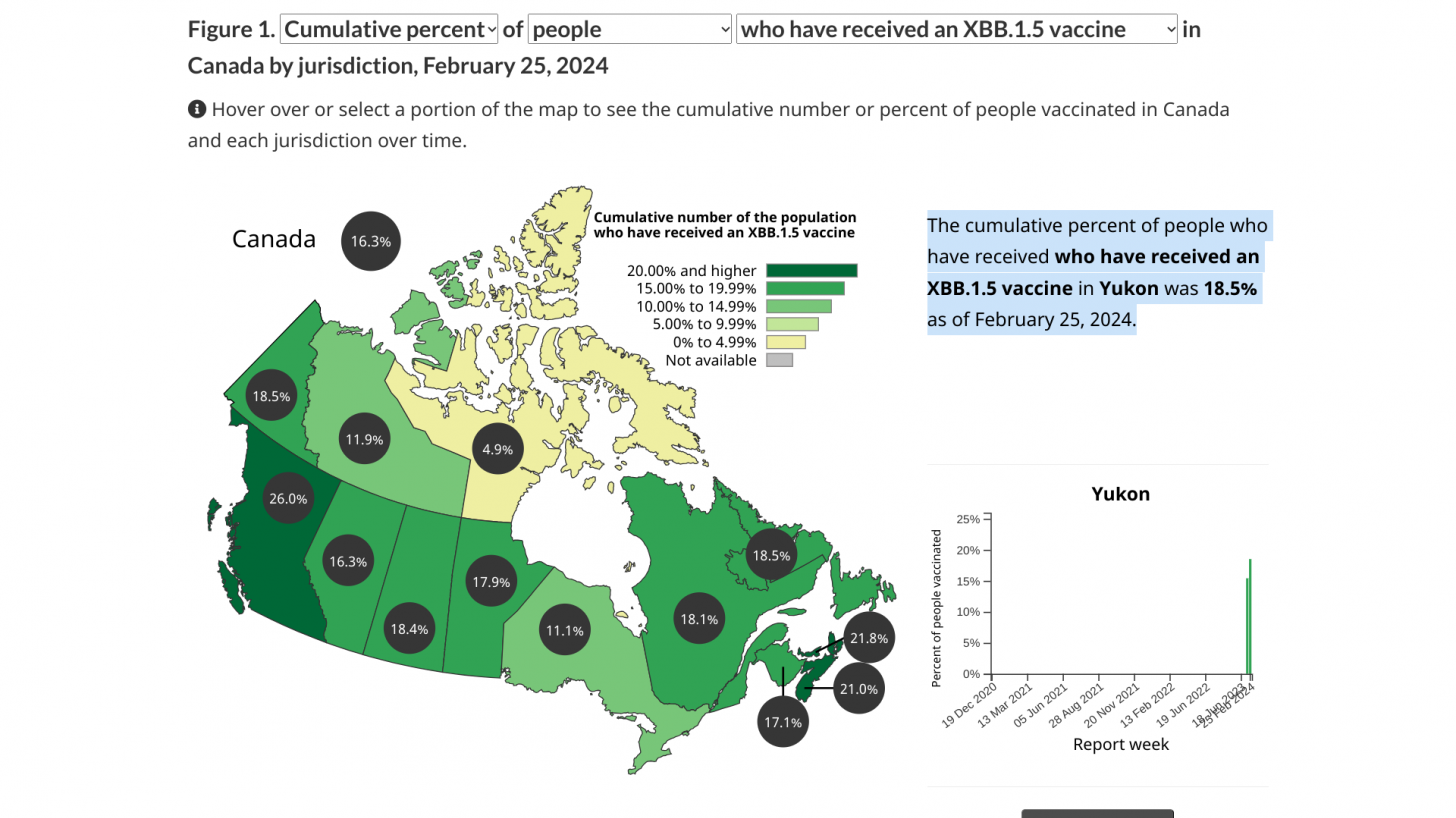
COVID-19 vaccinations began in Canada on December 14, 2020.
As of February 25, 2024, about 17% of Canadians had received an XBB.1.5 vaccine.
The Public Health Agency of Canada has distributed the protein-based vaccine across the regions, and provincial public health programs can advise on specific availabilities.
Two messenger ribonucleic acid (mRNA)-based COVID-19 vaccines remain available in Canada.
Data from clinical trials continue to show broad neutralization responses to currently circulating forward-drift variants, including JN.1 and JN.,4 for our protein-based non-mRNA COVID-19 vaccine while maintaining a favorable side effect profile. Novavax says peer-reviewed effectiveness data is being published to show how immune responses in clinical trials translate into COVID-19 prevention in the real world.
Our Trust Standards: Medical Advisory Committee
























