Will the U.S. FDA Approve Brazil's Dengue Vaccine
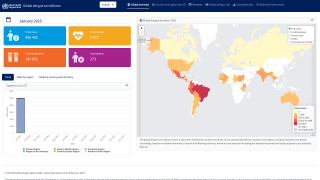
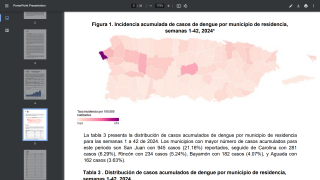
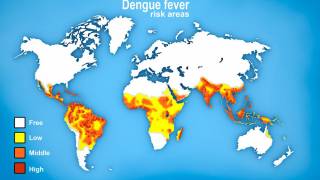
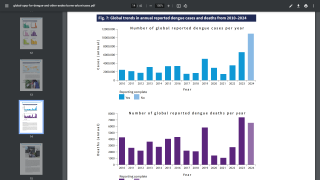
With over 12.7 million dengue virus cases and 7,828 related fatalities in 2024, countries throughout the Region of the Americas are seeking access to innovative vaccines.
The United States is one of those countries needing a dengue vaccine.
To potentially meet demand next year, the Butantan Institute today announced that it submitted the last batch of documents required for the registration of its dengue vaccine candidate (Butantan-DV) to Brazil's National Health Surveillance Agency (Anvisa).
With this, the Institute completed the submission of information. This phased evaluation tends to speed up the registration release process.
Butantan-DV, if approved, will be the world's first single-dose vaccine against dengue.
"The fact that the Butantan Institute can contribute with the world's first single-dose vaccine against dengue shows that it is worth investing in research conducted in Brazil and in the internal development of immunobiologicals. We will wait and respect all Anvisa procedures. But we are confident in the results that will come," commented Esper Kallás, director of the Butantan Institute, in a press release on December 16, 2024.
This announcement does not mean Butantan-DV will be available to Brazilians immediately after registration approval. If Anvisa's registration opinion is positive, the Institute must request price approval from the Chamber of Drug Market Regulation, which sets the price of new drugs in Brazil.
After this assessment, the National Commission for the Incorporation of Technologies into the Unified Health System will study the possibility of incorporating the vaccine into the local system.
From an efficacy perspective, Butantan-DV is very protective.
The vaccine's clinical studies were completed in June 2024, when the last participant completed five years of follow-up. Recently, results from phase 3 of the clinical trial, published in The Lancet Infectious Diseases, showed 89% protection against severe dengue and prolonged efficacy and safety for up to five years.
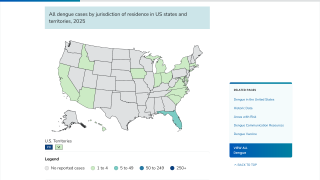
In the U.S., dengue vaccine access has been disrupted in 2024.
The one approved dengue vaccine has been withdrawn from the market, and Takeda withdrew its application to the U.S. FDA to approve its dengue vaccine.
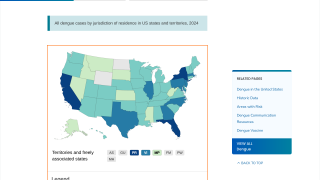
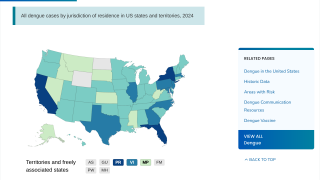
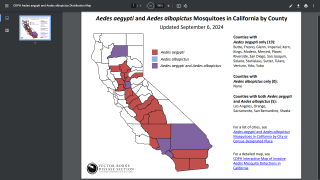
As of December 17, 2024, people living in areas reporting locally acquired dengue, such as Miami, Los Angeles, and San Juan, Puerto Rico, do not have access to preventive vaccines.
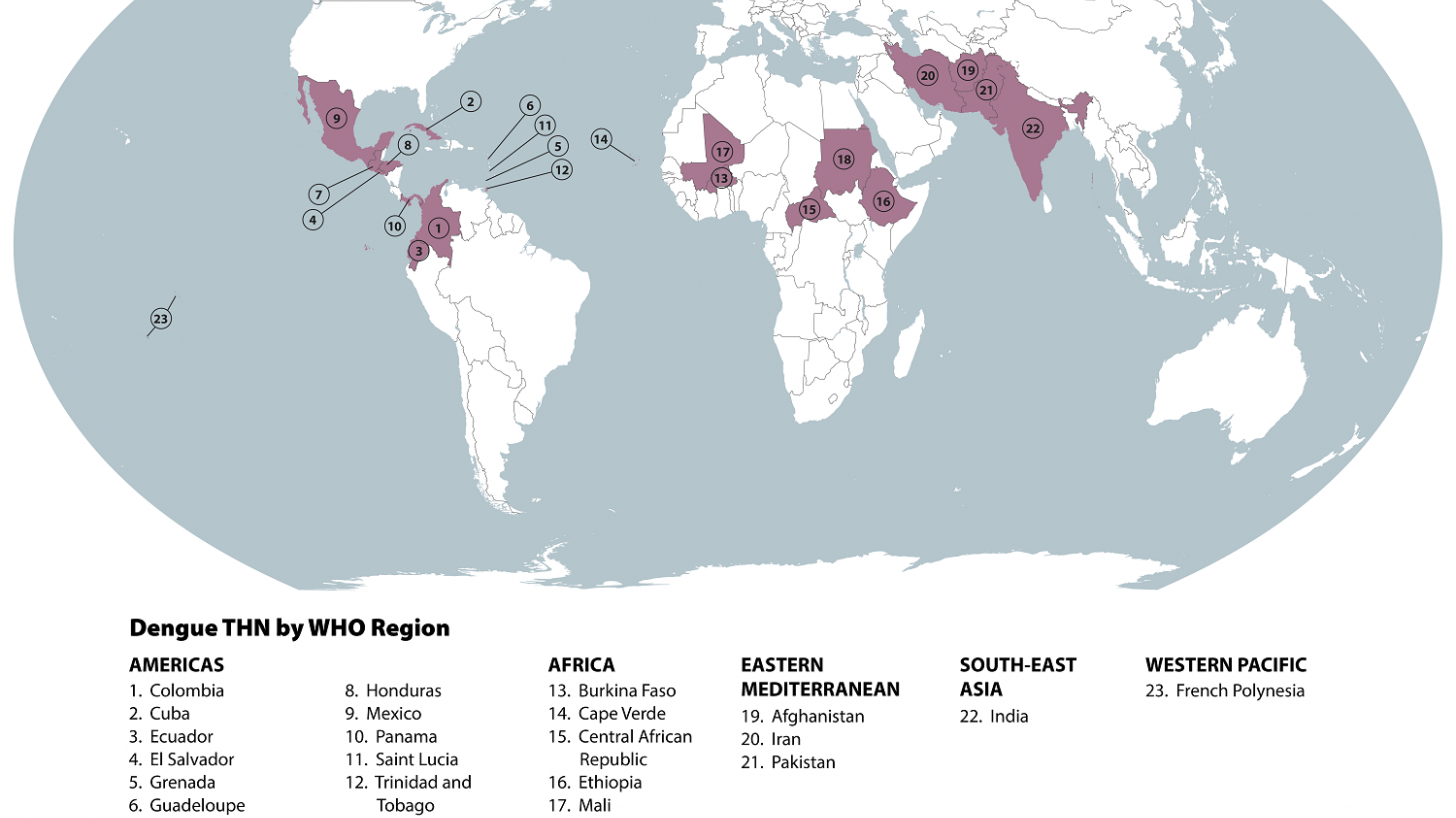
Furthermore, the CDC's updated Dengue Global Travel Health Advisory does not include a disease prevention option for travelers to 23 countries.
Many international travelers hope governments will approve next-generation dengue vaccines and candidates in 2025, potentially increasing access.
Our Trust Standards: Medical Advisory Committee