Vaccination Prevents Most Chikungunya Cases

The Lancet Infectious Diseases recently published a commentary emphasizing that the chikungunya virus is one of the most neglected arboviral diseases globally, with cases detected in 110 countries.
Chikungunya's impact on people's health has been significant.
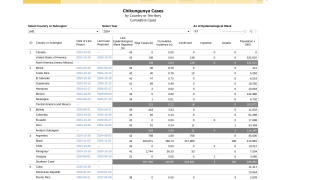
Public health authorities in the Region of the Americas have reported approximately 3.7 million chikungunya cases over the past decade. In the United States, this mosquito-transmitted disease is often diagnosed in international travelers returning from chikungunya endemic areas such as Brazil and the Caribbean.
In the U.S. as of August 3, 2024 (Week 31), including Territories and non-U.S. residents, the U.S. Centers for Disease Control and Prevention (CDC) reported 56 chikungunya cases. From 2006 to 2023, 4,590 travel-related chikungunya cases were reported in the U.S., in areas such as southeast Florida.
According to new results from a phase 3b clinical trial, the newly approved IXCHIQ® vaccine offers people significant protection against chikungunya disease.
The Lancet published results on August 12, 2024, showing that strong seroprotection was observed in one year (98·9% [356 of 360 assessable participants; 97·2–99·7]) and two years (96·8% [306 of 316; 94·3–98·5]) after vaccination.
Protection was very similar between those aged 18 and 64 (at one year: 98·7% [303 of 307; 96·7–99·6]; at two years: 96·6% [256 of 265; 93·7–98·4]).
And those aged 65 years and older (at one year: 100% [53 of 53; 93·3–100]; at two years: 98·0% [50 of 51; 89·6–100]) at each time point.
No adverse events of special interest were ongoing at the time of transition. Ten serious adverse events occurred in nine (2%) participants between the 6-month and 2-year time points, including one death (due to drug overdose) that was determined not to be related to IXCHIQ.
These researchers stated, 'after a single IXCHIQ vaccination, chikungunya virus-neutralizing antibodies above the threshold were considered to be protectively persisted for up to 2 years, and there were no long-term serious adverse events related to vaccination. Furthermore, IXCHIQ is an efficient and safe intervention that offers high seroprotection against chikungunya virus infection, a virus likely to spread globally with an urgent demand for long-lasting prophylaxis.'
Various countries, including Canada, Europe, and the U.S., have approved IXCHIQ.
The CDC suggests that international travelers speak with a travel vaccine advisor before visiting chikungunya outbreak areas and learn about its symptoms, which include fever and severe joint pain.
IXCHIQ was approved under U.S. FDA-accelerated approval based on anti-chikungunya neutralizing antibody titers. Valneva's IXCHIQ vaccine program received FDA Fast Track (2018) and Breakthrough Therapy designations (2021).
The funding for this study was provided by the vaccine's producer, Valneva SE, the Coalition for Epidemic Preparedness Innovation, and EU Horizon 2020.
Our Trust Standards: Medical Advisory Committee