Chikungunya Vaccine Also Protects People from Alphaviruses

As the science of virology continues evolving, discovering additional benefits from approved vaccines is a real advantage.
When the first chikungunya virus (CHIKV) vaccine was licensed in the U.S., Europe, and Canada, various research studies and government agencies concluded that Valneva SE's U.S. FDA-approved IXCHIQ® (VLA1553) vaccine demonstrated over 96% response rate after receiving a single administration, and most study participants maintained very high protection six months after vaccination.
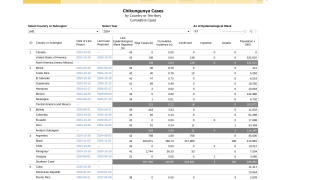
These findings were well received in 2024, as the number of countries in the region of the Americas confirming CHKV outbreaks has reached 13.
As of September 1, 2024, over 383,000 CHIKV cases and 162 related deaths have been reported this year.
And according to a study published by the journal MDPI in early August 2024, IXCHIQ vaccination offers protection against alphaviruses that co-circulate with CHIKV.
These researchers wrote, 'the significant questions remain regarding the potential of IXCHIQ to confer cross-protection for populations exposed to them.
This study characterized the cross-neutralizing antibody (nAb) responses against heterotypic CHIKV and additional arthritogenic alphaviruses in individuals at one month, six months, and one-year post-IXCHIQ vaccination.
At least 27 alphaviruses and 68 flaviviruses have been recognized, approximately one-third of which are medically important human pathogens. Alphaviruses and flaviviruses can cause various syndromes, ranging from benign febrile illnesses to severe systemic diseases with hemorrhagic manifestations or organ involvement.
They characterized nAbs against CHIKV strains LR2006, 181/25, and a 2021 isolate from Tocantins, Brazil, as well as O’nyong-nyong virus, Mayaro virus, and Ross River virus (RRV).
IXCHIQ elicited 100% seroconversion to each alphavirus, except RRV, which had 83.3% seroconversion of vaccinees. The cross-neutralizing antibody potency decreased with increasing genetic distance from CHIKV.
These researchers wrote, "Our work reveals the first report of cross-neutralizing antibodies induced by the licensed vaccine IXCHIQ. We also have evidence that these antibodies persist at one-year post-vaccination and share potency and breadth features consistent with those seen following natural infection."
Additionally, "our study directly compares these vaccinee responses to the cross-neutralizing antibodies generated in response to CHIKV infection. It shows that IXCHIQ elicits neutralizing antibody populations similar in potency and breadth to antibodies elicited by natural CHIKV infection."
These researchers concluded that this finding "may have important implications for individuals susceptible to alphavirus co-circulation in regions of potential vaccine rollout."
As of September 2024, the IXCHIQ vaccine is offered at various clinics, pharmacies, and travel vaccine specialists in the United States, such as Passport Health USA.
Our Trust Standards: Medical Advisory Committee