Concomitant of IPV and nOPV2 Vaccines Suggested During Polio Outbreaks

The WHO Executive Board recently published a report following its 156th session, which provides an update on progress toward fully implementing and financing the Polio Eradication Strategy and the Polio Transition Framework.
Based on the September 2024 Strategic Advisory Group of Experts on Immunization (SAGE) meeting, the report was published on December 11, 2024.
Within this report, the Polio Oversight Board extended the Strategy by three additional years.
This extension shifts the timeline for eradicating wild poliovirus type 1 to the end of 2027 and the goal for certifying the elimination of circulating vaccine-derived poliovirus type 2 (cVDPV2) to the end of 2029.
Additionally, the Global Polio Eradication Initiative continues to support integration activities across the program in accordance with the Polio Oversight Board-approved integration work plan. The plan focuses on strengthening routine immunization, integrating service delivery in seven priority countries, and campaign-based integration across all polio-affected countries.
These efforts include the emergency response to returnees to Afghanistan, integrated service delivery in Pakistan, and multi-antigen campaigns in 20 countries.
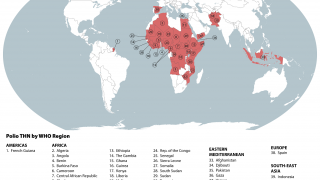
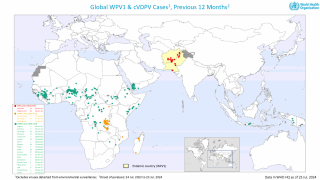
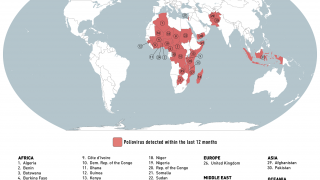
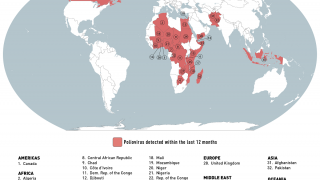
Between September and November 2024, poliovirus in wastewater samples was detected in Germany and the United Kingdom.
In the UK, cVDPV2 was isolated from an environmental sample collected in November from Leeds and from environmental samples collected from London and East Worthing.
In Germany, six additional cVDPV2 isolates were confirmed from environmental samples collected in November from Bonn, Dresden, Duesseldorf, Hamburg, Cologne, and Mainz. All of these detected isolates were linked to the strain originating in Nigeria.
"VDPVs are a major challenge for eradicating polio. These viruses were first noticed during a 2000 outbreak in Hispaniola. Between 2017 and February 2024, VDPV2 has been reported in 49 countries, especially in sub-Saharan Africa, Syria, Afghanistan, Pakistan, and other countries," commented Duellyn Pandis, DNP, APRN, FNP-C.
"High-income and many middle-income countries have eliminated polio with the inactivated polio vaccine (IPV), but achieving similar success in crowded, unsanitary areas remains difficult. Over 40 countries have seen the reintroduction of wild polio or spread of vaccine-derived polio due to low population immunity."
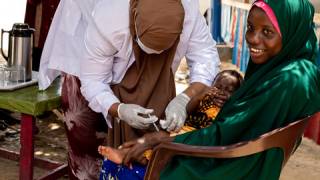
"The best course to prevent infection from polio is with vaccination. The United States uses a killed version of the polio vaccine IPOL produced by Sanofi Aventis. It protects infants (6 weeks of age), children, and adults against poliomyelitis caused by poliovirus Types 1, 2, and 3. If traveling to a country with a known outbreak, ensure the full series of polio vaccines have been completed."
"If it is more than 10 years since the last polio vaccine, an adult booster is indicated," added Pandis, President and CEO of Passport Health - Tampa.
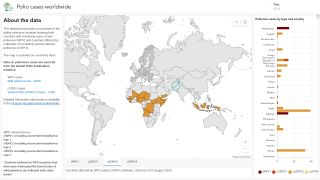
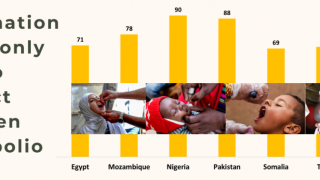
To date, the Global Polio Eradication Initiative has contributed oral polio vaccines (OPV) for ten countries as part of the "Big Catch-Up" initiative, which aims to reduce the number of zero-dose children and increase routine immunization rates.
Among other factors, the SAGE reviewed the global polio epidemiology and expressed support for the ongoing development of the bOPV vaccine cessation policy framework. In some situations, OPVs can mutate and regain virulence, leading to vaccine-derived poliovirus, paralyzing an unvaccinated person.
SAGE also recommended that, where feasible, the concomitant use of IPV and type 2 novel oral polio (nOPV2) be used for initial outbreak response vaccination campaigns.
The nOPV2 vaccine has been 'triple-locked' using genetic engineering to prevent it from becoming harmful and producing a mutation. As a result, nOPV2 is reported to be more genetically stable than previous oral polio vaccines.
As of early December 2024, over 1.1 billion doses of the nOPV2 vaccine have been administered worldwide.
Furthermore, in response to recently confirmed poliovirus detections, a WHO Committee unanimously agreed as of December 3, 2024, that the risk of the international spread of poliovirus remains a Public Health Emergency of International Concern and recommended the extension of Temporary Recommendations for three months into 2025.
This WHO recommendation includes ensuring all visitors to polio-endemic areas are fully vaccinated, and some adults may need a one-time polio vaccine booster.
In the U.S., the IPV vaccine is available at health clinics and pharmacies, but not the nOPV2 vaccine.
Our Trust Standards: Medical Advisory Committee