Sexually Transmitted Disease Epidemic Accelerates
The World Health Organization, the U.S. National Institutes of Health, and global partners have published a comprehensive roadmap for developing new vaccines against sexually transmitted infections (STIs).
Since its publication in 2014, vaccines for Mpox, human papillomavirus, and hepatitis have become readily available in the U.S.
However, clinical advances for preventing herpes, HIV, chlamydia, gonorrhea, Epstein-Barr, and syphilis remain unrealized. These scientific shortcomings leave many people exposed to lifelong diseases.
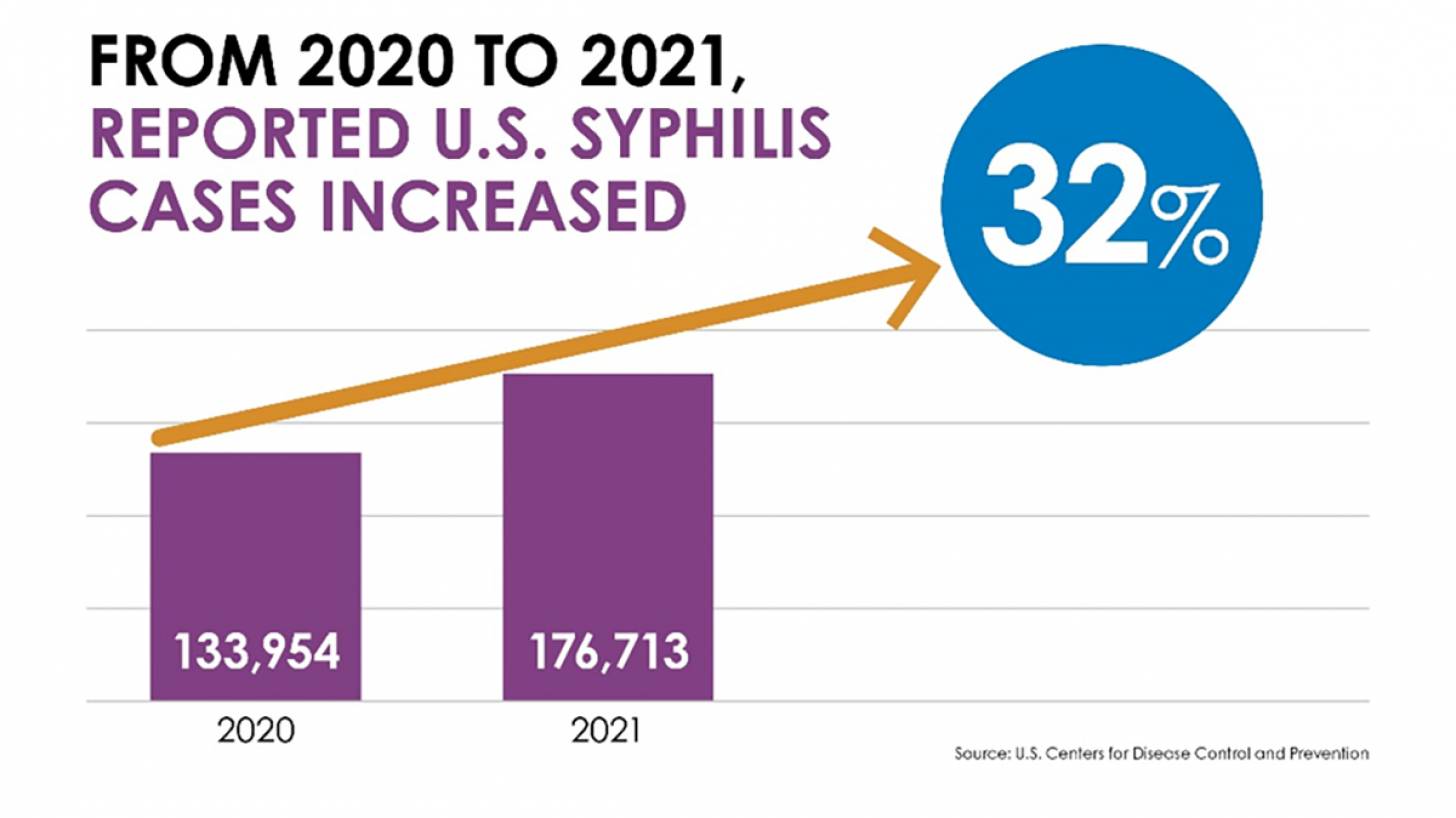
For example, reported cases of sexually transmitted infections, chlamydia, gonorrhea, and syphilis increased between 2020 and 2021, reaching more than 2.5 million reported cases, according to U.S. Centers for Disease Control and Prevention (CDC) surveillance data.
Specifically, as of April 11, 2023, syphilis rates surged the most, increasing by nearly 32% for combined stages of the infection.
Among the CDC's syphilis data, pregnant women have been measurably impacted, as cases of congenital syphilis resulted in 220 stillbirths and infant deaths.
To reverse this unfortunate trend, the CDC recently called for more healthcare, industry, and public health groups to contribute to prevention and innovation efforts.
These efforts include exploring new interventions, such as innovative vaccines or post-exposure prophylaxis strategies to prevent herpes, HIV, and gonorrhea transmissions.
Leandro Mena, M.D., MPH, Director of CDC's Division of STD Prevention, wrote, "The STI epidemic shows no signs of slowing. The reasons for the ongoing increases are multifaceted – and so are the solutions."
"For the first time in decades, we're seeing promising new STI interventions on the horizon, but these alone will not solve this epidemic."
"It will take many of us working together to effectively use new and existing tools, to increase access to quality sexual healthcare services for more people, and to encourage ongoing innovation and prioritization of STI prevention and treatment in this country."
To support these vaccine development efforts, the U.S. government announced on March 1, 2023, it is seeking applicants who can develop advanced vaccines that have limited candidates in the product development pipeline through the new request for applications Sexually Transmitted Infections Cooperative Research Centers Vaccine Development.
While U.S. NIAID's Division of Microbiology and Infectious Diseases has made substantial investments in STI vaccine development, this new initiative focuses on advanced vaccine development for STI pathogens with limited candidates in the product development pipeline, such as Neisseria gonorrhoeae, Chlamydia trachomatis, and Treponema pallidum.
A significant, recent scientific advancement has been launching mRNA vaccines targeting herpes simplex virus (HSV) prevention.
Over the past year, the mRNA-1608 and BNT163 HSV vaccine candidates launched clinical trials.
These early-stage studies may take years to complete, but deploying this world-class vaccine technology offers hope to many people.
Our Trust Standards: Medical Advisory Committee
























