repRNA Vaccination In Utero Transfers Protective Antibodies Against Zika

Researchers recently reported newly developed mRNA vaccines against the Zika virus, and HIV-1 produced strong antibody responses that transferred from pregnant rabbits to their offspring.
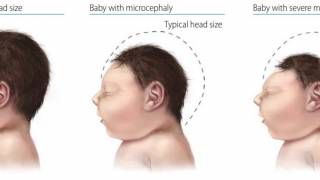
These two pathogens play a significant role in causing infections in newborns after mother-to-child transmission.
Published in the journal Molecular Therapy on March 24, 2023, the study's results support further development of their vaccine platform, LIONTM/repRNA, for maternal and neonatal settings to protect against mother-to-child transmission of pathogens in animals and humans.
"Preventing mother-to-child transmission is a major goal for reducing disease burden in newborns," says senior author Amit Khandhar, a material scientist at HDT Bio Corp, in a press release.
"With mRNA vaccines attracting global attention, there is a need to evaluate their safety and immunogenicity in preclinical models that inform maternal and childhood vaccination."
Khandhar collaborated with Herman Staats of Duke University School of Medicine and Noah Sather of Seattle Children's Research Institute to evaluate self-amplifying replicon (repRNA) vaccines.
The repRNA vaccines encode viral enzymes that amplify the expression of a gene of interest by 10- to 100-fold over non-replicating mRNA, providing dosing and manufacturing advantages.
The researchers delivered the vaccines with their clinical-stage LIONTM nanoparticle formulation in pregnant rabbits using the Zika virus and HIV-1 as model disease targets.
The proprietary LIONTM delivery technology is a stable oil-in-water nanoparticle emulsion that electrostatically binds and protects nucleic acids, in contrast to lipid nanoparticle formulations, which encapsulate RNA.
Because LIONTM is stored independently of repRNA, it has plug-and-play functionality, allowing for rapid evaluation of new repRNA vaccine constructs such as those recently developed to address emerging SARS-CoV-2 variants.
The results showed that repRNA immunization at a relatively high dose was well tolerated and had no detrimental impact on litter size. The LIONTM/repRNA vaccines also triggered robust antigen-specific antibody responses in pregnant adult rabbits that were likely passively transferred to offspring in utero
"While the strong correlation in both the magnitude and quality of antibody levels between mothers and newborns suggests that the antibodies detected in kits were likely acquired passively from mothers, we cannot completely rule out the possibility that the vaccine administered to mothers may itself distribute to kits and actively induce antibody responses," Khandhar says.
The researchers also found that the timing of maternal vaccination was critical for maximizing antibody transfer, and subsequent vaccination in newborns maintained elevated antibody levels compared to no vaccination. In addition to optimized maternal vaccination timing, active immunization in newborns might be required for maintaining overall antibody responses in infants after birth.
The authors say more research is needed to determine whether RNA-based maternal vaccines can afford protection against infection by mother-to-child transmission.
"For instance, the immunization intervals we used were not optimized and will likely not translate to humans due to differences in the gestation periods between rabbits and humans," Khandhar says.
"Further studies will be needed to test boosting intervals and the durability of antibody responses to maximize passive antibody transfer to newborns."
"Lastly, additional studies designed to measure safety signals in maternal and neonatal models will be needed before advancing to clinical evaluation."
The National Institute of Allergy and Infectious Diseases partly supported this research. In addition, several co-authors have an equity interest in HDT Bio and are listed as inventors on patents covering the LIONTM formulation.
Note: As of March 30, 2023, two mRNA vaccine candidates are conducting clinical trials focused on the Zika virus.
Our Trust Standards: Medical Advisory Committee