Zika Testing Guidelines Updated

Since the Zika virus outbreaks of 2016, the number of reported Zika cases in the Americas has significantly declined.
According to the US Centers for Disease Control and Prevention (CDC), the last confirmed cases of a nucleic acid amplification test (NAAT) regarding locally-acquired Zika in the continental USA was in March 2018.
But, travel-related Zika cases have been reported in the States of California (30) and Florida (36), as of November 21, 2019.
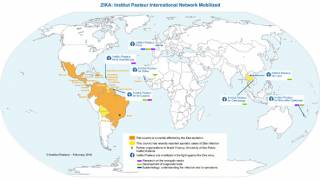
Furthermore, the Pan American Health Organization reported 4,299 Zika virus cases in the Americas during 2019.
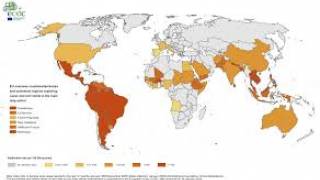
And the risk of acquiring Zika while traveling in Europe has recently been reported.
On October 31st, French authorities reported a 3rd autochthonous Zika case in the Var department of France.
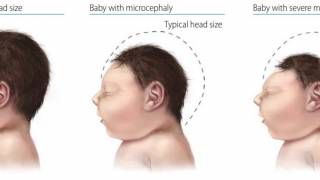
The Zika virus disease has been reported to cause fetal abnormalities, such as microcephaly and Guillain-Barre syndrome in adults.
The Zika virus is transmitted to people primarily through the bite of infected Aedes aegypti or Aedes albopictus mosquitoes. An Aedes mosquito can only transmit the Zika virus after it bites a person who has the virus in their blood.
Furthermore, various studies have shown that Zika can also be sexually transmitted, says the CDC.
Given the current global arboviral epidemiological situation, the CDC updated its Zika testing guidance on November 18, 2019. This revised guidance is as follows:
Asymptomatic pregnant women:
- For asymptomatic pregnant persons living in or with recent travel to the U.S. and its territories, routine Zika virus testing is NOT currently recommended.
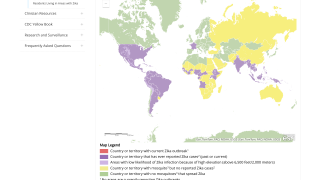
- For asymptomatic pregnant women living in or with recent travel to an area with risk of Zika (purple areas) outside the U.S. and its territories, Zika virus testing is NOT routinely recommended, but NAAT testing may still be considered.
- Zika virus serologic testing is NOT recommended for asymptomatic pregnant women. Zika IgM antibodies can persist for months to years following infection. Therefore, detecting Zika IgM antibodies might not indicate a recent infection.
- There is notable cross-reactivity between dengue IgM and Zika IgM antibodies in serologic tests. Antibodies generated by a recent dengue virus infection can cause the Zika IgM to be falsely positive.
Symptomatic pregnant patients:
- Specimens should be collected as soon as possible after symptom onset for symptomatic pregnant persons living or with recent travel to areas with a risk of Zika.
- The following diagnostic testing should be performed at the same time. Zika virus NAAT testing on a serum specimen, and Zika virus NAAT on a urine specimen.
- Zika virus IgM testing is NOT recommended for symptomatic pregnant women.
- If the Zika NAAT is positive on a single specimen, the Zika NAAT should be repeated on newly extracted RNA from the same specimen to rule out false-positive Zika NAAT results.
Pregnant women who have a fetus with prenatal ultrasound findings consistent with congenital Zika virus infection who live in or traveled to areas with a risk of Zika during her pregnancy:
- Zika virus NAAT and IgM testing should be performed on maternal serum and NAAT on maternal urine.
- If the Zika virus NAATs are negative and the IgM is positive, confirmatory PRNTs should be performed against Zika and dengue.
- If amniocentesis is being performed as part of clinical care, Zika virus NAAT testing of amniocentesis specimens should also be performed and results interpreted within the context of the limitations of amniotic fluid testing.
- It is unknown how sensitive or specific RNA NAAT testing of amniotic fluid is for congenital Zika virus infection or what proportion of infants born after infection will have abnormalities.
- The testing of placental and fetal tissues may also be considered.
Symptomatic non-pregnant patients should refer to testing guidance for dengue.
- Zika testing is NOT currently recommended for this group based on the current epidemiology of these viruses.
- As per previous guidance, asymptomatic non-pregnant patients should NOT be tested for dengue or Zika viruses
- Zika virus testing should NOT be performed as part of preconception screening.
This CDC page will be updated as needed to communicate new CDC testing guidelines based on the changing epidemiology of Zika.
Zika virus news is published by Zika News.
Our Trust Standards: Medical Advisory Committee