Marburg Vaccine Candidate Receives HHS Funding

To increase national health security against biothreats, the U.S. Department of Health and Human Services (HHS) announced it will begin co-developing a vaccine candidate against the Marburg virus.
In addition to the threat of a naturally occurring infection, the Marburg virus is deemed a potential bioterrorism threat by the U.S. Department of Homeland Security.
Currently, there is not a licensed vaccine for Marburg.
The Biomedical Advanced Research and Development Authority (BARDA), part of the HHS Office of the Assistant Secretary for Preparedness and Response, awarded an initial 2-year, $10 million contract to Public Health Vaccines LLC, to begin development of a vaccine to protect against Marburg infection.
“This vaccine candidate is the first BARDA has funded against the Marburg virus, and it is an important step toward meeting an urgent public health and biodefense need,” said BARDA Director Rick Bright, Ph.D.
“We will leverage our experience in establishing public-private partnerships that bring results that are critical to saving lives and protecting Americans – and possibly people across the globe – from health security threats."
The Public Health Agency of Canada initially developed the vaccine and licensed it to Public Health Vaccines LLC. This approach is similar to the one Merck & Co. used to develop its Ebola vaccine.
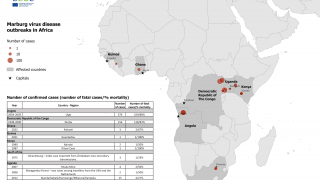
The Marburg virus was recognized in 1967 and since then multiple outbreaks have occurred with high mortality rates, most recently in 2017.
The Marburg virus is part of the family of hemorrhagic fever viruses that includes Ebola.
Marburg hemorrhagic fever (Marburg HF) is a rare but severe hemorrhagic fever which affects both humans and non-human primates. Marburg HF is caused by Marburg virus.
Marburg HF typically appears in sporadic outbreaks throughout Africa; laboratory-confirmed cases have been reported in Uganda, Zimbabwe, the Democratic Republic of the Congo, Kenya, Angola, and South Africa, says the CDC.
Recent Marburg virus articles:
- Marburg Virus Reported in Sierra Leone Bats
- Marburg Virus Vaccine Candidate Launches Phase 1 Study In Maryland
The mission of the Office of the Assistant Secretary for Preparedness and Response (ASPR) is to save lives and protect Americans from 21st-century health security threats. Within ASPR, BARDA invests in the advanced research and development, acquisition, and manufacturing of medical countermeasures – vaccines, drugs, therapeutics, diagnostic tools, and non-pharmaceutical products needed to combat health security threats.
To date, 43 BARDA-supported products have achieved regulatory approval, licensure or clearance. BARDA accepts proposals for the advanced development of medical countermeasures through the Broad Agency Announcement, BARDA-BAA-18-100-SOL-00003.
Click here for more information on partnering with BARDA on developing medical countermeasures.
Our Trust Standards: Medical Advisory Committee