700 Marburg Vaccine Candidates Heading to Rwanda

The Sabin Vaccine Institute announced it has provided 700 of its Marburg Virus Disease (MVD) vaccine candidates (PHV01) to the Republic of Rwanda to support the ongoing disease outbreak response.
As of October 5, 2024, the initial vaccine shipment will be used in a trial targeting frontline workers in Rwanda, including healthcare professionals.
Pending a request from Rwandan officials and authorization from others, Sabin plans to supply additional vaccines.
Sabin has agreed with the Rwanda Biomedical Centre to provide these investigational doses for the Phase 2 rapid response open-label study. Per the approved protocol, adults will be dosed at six clinical trial sites in Rwanda.
Sabin's single-dose vaccine is based on the cAd3 platform and is conducting Phase 2 trials in Uganda and Kenya with no reported safety concerns.
Results from earlier Phase 1 clinical trials and nonclinical studies indicate that PHV01 is safe and elicits rapid, robust immune responses.
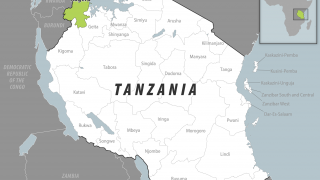
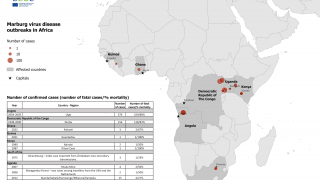
Rwanda declared its first MVD outbreak on September 27, 2024, and as of October 5, 2024, it had infected 46 people and claimed 12 lives. While most cases are among health workers in two facilities in Kigali, the capital, a smaller number are spread across a few other districts.
As of October 5, 2024, there is no approved vaccine available. While Sabin's vaccine is the first to be deployed in Rwanda, other Marburg vaccine candidates are accelerating clinical studies, such as the following:
Soligenix, Inc. MarVax™ is a subunit protein vaccine.
Researchers at the University of Oxford are developing the ChAdOx1 Marburg vaccine candidate.
IAVI's single-dose rVSVΔG-MARV-GP vaccine candidate against Marburg virus.
Marburg virus disease is a member of the Filoviridae family of viruses and is a severe but rare viral disease that has infected humans since 1967.
Our Trust Standards: Medical Advisory Committee