Europe Adds Second Chikungunya Vaccine

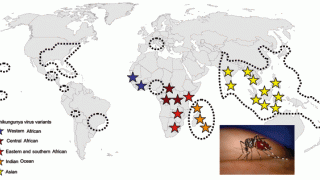
While the global Chikungunya outbreak in 2024 impacted over 100 countries, most European countries reported very few Indigenous (locally) acquired cases.
However, according to the European CDC, the geographic expansion of Ae. albopictus mosquitoes to more temperate regions in Europe have increased the risk of arboviral disease outbreaks in 2025.
Last year, reports from the health ministries of France, Italy, and Spain showed that the number of travel-related and local Chikungunya cases could accelerate in 2025.
For example, outbreaks in France's La Réunion Étang-Salé and Sheunon ravine districts highlighted this health concern in 2024. Due to the increase and dispersion of Chikungunya cases this year, La Réunion triggered a level 3 alert on January 13, 2025, corresponding to a low-intensity epidemic.
To combat the spread of this mosquito-transmitted disease, Valneva SE's IXCHIQ® Chikungunya vaccine was approved in Europe and elsewhere. This innovative vaccine delivers strong protection with few side effects.
According to the European Medicines Agency (EMA) announcement today, a second-generation Chikungunya vaccine may soon become available throughout Europe.
Bavarian Nordic A/S has announced that the EMA's Committee for Medicinal Products for Human Use has adopted a positive opinion recommending marketing authorization for VIMKUNYA® (CHIKV VLP vaccine), an active immunization for the prevention of disease caused by the chikungunya virus in individuals 12 years of age and older. Studies have shown that VLP vaccines are highly immunogenic and have a proven safety record.
The European Commission, which grants central marketing authorizations in the European Union, will review the recommendation and is expected to make a final decision in the coming months. If granted, the marketing authorization of VIMKUNYA would be valid in all EU member states, as well as in Iceland, Liechtenstein, and Norway.
"The recommendation of our chikungunya vaccine for approval in Europe marks a huge milestone in our efforts to deliver protection against this debilitating disease and, once approved, would represent a significant contribution to expanding the availability of vaccines to a broader population, including adolescents aged 12-17 years," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on January 31, 2025.
Bavarian Nordic also confirmed it will now also submit a Marketing Authorization Application to the United Kingdom, with the potential approval of VIMKUNYA in the first half of 2025.
The vaccine is also under priority review with the U.S. Food and Drug Administration, with a Prescription Drug User Fee Act action date of February 14, 2025.
While seldom discussed, Chikungunya disease has been found to cause substantive health issues, especially in Brazil.
In a recent original article published by The Journal of Pediatrics, researchers concluded abnormal neurodevelopmental outcomes were observed in both infected and uninfected children who had been exposed to CHIKV either during pregnancy or around the time of birth.
Adding a second Chikungunya vaccine in Europe and elsewhere and expanding clinic and pharmacy access, more international travelers should be protected from this disease in 2025.
Our Trust Standards: Medical Advisory Committee