Pre-Pregnancy DNA Vaccination Protected Monkeys From Zika

According to a new study by researchers at the University of California, Davis and the NIH concluded a Zika virus vaccine given to women prior to becoming pregnant may help prevent damaging sequelae in future offspring.
This study published in Science Translational Medicine on December 18, 2019, assessed fetal protection of immunized nonhuman primates with the DNA vaccine VRC5283 before conception, then performed multiple Zika virus challenges in the first few months of pregnancy.
Compared to unvaccinated controls, animals vaccinated with VRC5283 had reduced viremia in terms of both magnitude and duration.
And, early fetal loss and fetal pathology were accordingly reduced.
This is important news since a Zika virus infection of pregnant women is associated with adverse fetal effects, clinically termed as Congenital Zika Syndrome.
To more accurately mimic how this Zika vaccine candidate would be used, these UC Davis researchers immunized macaques before conception and then exposed them repeatedly to ZIKV during early and mid-gestation.
In comparison to unimmunized animals, vaccinated animals had a significant reduction in peak magnitude and duration of maternal viremia, early fetal loss, fetal infection, and placental and fetal brain pathology.
Vaccine-induced neutralizing antibody titers on the day of first Zika virus exposure were negatively associated with the magnitude of maternal viremia, and the absence of prolonged viremia was associated with better fetal outcomes.
‘These results suggest that the VRC5283 vaccine may prevent mother-to-fetus transmission of Zika virus in humans as well,’ said Koen Van Rompay, Ph.D., a virologist at the California National Primate Research Center, at UC Davis, in a related press release.
These data support further clinical development of Zika vaccine strategies to protect against negative fetal outcomes.
Dr. Van Rompay added ‘The candidate vaccine is currently in global phase IIb trials to test its safety and ability to elicit an immune response in humans.
Additional clinical trials to determine efficacy would be needed to support the licensure of the VRC5283 vaccine.
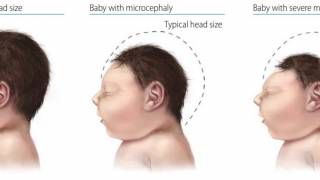
UC Davis researchers are also looking at how the Zika virus affects the development of young macaques.
Rhesus macaques do not develop the same head malformation (microcephaly) seen in some human infants.
Dr. Van Rompay said, ‘It is thought that there could be many more infants exposed to Zika virus before birth who may show more subtle developmental deficits.’
By carefully monitoring macaques that were exposed to the virus before birth for a long time after birth, the UC Davis researchers hope to pick up on other problems that might also show up in children.
The Zika virus is an arthropod-borne virus of the family Flaviviridae, genus Flavivirus. Although originally isolated in 1947, its pathogenesis was poorly known and very few documented infections were published until recently.
Recent Zika virus news
The investigational VRC-ZKADNA085-00-VP vaccine was developed by the Vaccine Research Center, NIAID, and is composed of a single closed-circular DNA plasmid that encodes the wild type precursor transmembrane M and envelope proteins from the H/PF/2013 strain of Zika virus.
This research was funded, in part, by these grants from the National Institutes of Health: R21-AI-129479-S; P51-OD-11107; R21-NS-104692; R21-HD-090856; R01-HD-098389; T32- CA009111 and by the NIAID Intramural Research Program.
Zika research news published by Zika News
Our Trust Standards: Medical Advisory Committee
- DNA vaccination before conception protects Zika virus–exposed pregnant macaques against prolonged viremia and improves fetal
- Safety and Immunogenicity of a Zika Virus DNA Vaccine, VRC-ZKADNA085-00-VP, in Healthy Adults
- Zika Vaccine Protects Fetus in Pregnant Monkeys
- DNA Vaccine Candidates For Zika Found Safe
- VRC 705: A Zika Virus DNA Vaccine in Healthy Adults and Adolescents (DNA)