Recombinant BCG Vaccine Becomes Available in the U.S.

The Bacillus Calmette-Guérin (BCG) is one of the most widely used vaccines worldwide, with about 4 billion doses administered over the past 100 years.
However, BCG is a biologic drug that uses benign bacteria, which makes it more complicated to manufacture than other drugs. This production limitation has led to decades-long vaccine shortages.
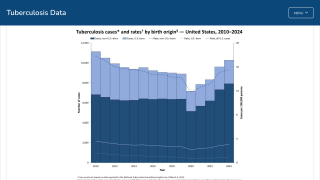
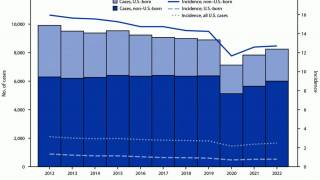
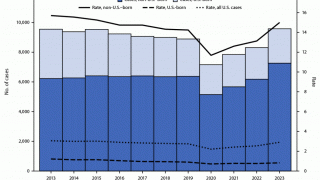
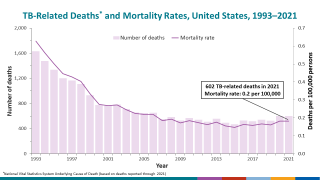
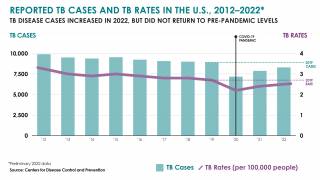
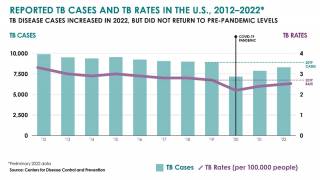
It has also been found to offer limited protection against diseases such as tuberculosis, an expanding health risk.
To resolve these shortcomings, ImmunityBio, Inc., today announced that U.S. Urology Partners, one of the nation's largest independent providers of urology and related specialty services, is one of the first providers to participate in ImmunityBio's Expanded Access Program (EAP) for the recombinant Bacillus Calmette-Guérin (rBCG) vaccine.
Serum Institute of India (SII), the largest manufacturer of BCG vaccines globally, has partnered with ImmunityBio to make this vaccine. Also, two gene modifications have been implemented in rBCG to improve its immunogenicity and safety compared to earlier strains and formulations of BCG.
Previously, Merck's TICE® BCG was the only vaccine available in the U.S., primarily used to prevent TB in children. Healthcare providers unable to source TICE BCG can access rBCG through the ImmunityBio EAP.
"It is gratifying to see the rapid uptake and interest in the rBCG EAP, which was authorized recently by the FDA," commented Dr. Patrick Soon-Shiong, Founder, Executive Chairman, and Global Chief Scientific Medical Officer of ImmunityBio, in a press release on March 13, 2025.
"Participation by a major national provider like U.S. Urology Partners is a testament to the critical importance of rBCG and the need for an alternative supply of this essential medicine."
Supply shortages of TICE BCG become a significant impediment to the treatment of bladder cancer patients. In a recent survey of 100 U.S. urologists, 57% indicated they could not treat patients in the last 12 months due to a lack of access to TICE BCG.
The SII is the world's largest vaccine manufacturer by volume. In European bladder cancer clinical trials, the rBCG vaccine (TUBERVAC-rBCG ) demonstrated potent immunogenicity with CD8+ and CD4+ T cell stimulation and improved safety compared to earlier BCG strains and formulations. It was approved in Europe in 2023.
rBCG vaccination induces an immune response in the bladder near the cancer cells, leading to cancer clearance in many patients.
Other countries, such as Brazil, are evaluating rBCG vaccines for use with children.
ImmunityBio has been awarded multiple patents covering the composition and methods of use for the combination of BCG plus ANKTIVA® in bladder cancer, which the U.S. FDA has approved.
In a 2024 study, ANKTIVA, in combination with BCG, achieved a 71% complete response rate in bladder cancer therapy.
Our Trust Standards: Medical Advisory Committee